The PCTPro-2000 - The Ultimate Tool for Gas Sorption Analysis
Karl J. Gross, Dr.
Hy-Energy, LLC
Introduction
Gas sorption analysis is important in many fields of materials science and consumer product development. Some examples of current hot areas of technology involving gas-solid (or gasliquid) interactions include the development of energy storage materials, improved catalysts for petrochemical processing, advanced pharmaceuticals and food industries. At present, there is tremendous interest in the development of innovative materials for the transition to renewable energy. The science of gas sorption has become particularly critical in the advancement of materials for storing fuel gases such as hydrogen or natural gas, as well as for sequestration of greenhouse gases. Some exciting prospects for the chemical storage of gases include high-surface area and nanomaterials (graphitic carbons, CNT, zeolites, metal-organic framework).1 In the field of hydrogen-storage, a host of novel materials are being developed, including high-pressure metalhydrides, light-weight complex hydrides, destabilized multicomponent chemical hydrides and amides, and other crossover materials such as ammonia borane encapsulated into mesoporous silica scaffolding.2
Of prime interest in the discovery of these and many other new materials is the characterization of gas absorption, adsorption and desorption kinetics, capacity, thermodynamic properties, as well as cycling performance of reversible materials. Also important is the ability to measure the tolerance of catalyst, chemicals, and consumer products to air, moisture and low-level contaminants. These wide-ranging requirements and the need to perform analysis at both a research and a production level (milligrams to kilograms) have driven the development of the PCTPro-2000, the ultimate tool for gas sorption analysis.
Careful analysis of physical and chemical interactions between gases and solids (or liquids) require extremely precise measurements. Several techniques are employed for gas sorption measurements the most common of these being gravimetric and volumetric methods. In volumetric methods the amount of gas sorbed is typically determined by a change in pressure within a calibrated volume containing the sample. In gravimetric methods, the amount of sorbed gas is determined by measuring the apparent mass change of the sample. In volumetric methods, the amount of gas sorbed is typically determined by a change in pressure within a calibrated volume containing the sample. We believe the volumetric method offers several distinct advantages over the gravimetric method, and of the volumetric instruments available, the PCTPro-2000 offers the best state-of-the-art measurements.
The Volumetric Method
One of the most common and versatile types of volumetric instruments is the Sieverts Apparatus. Simply put, a Sieverts Apparatus is an instrument containing two reservoirs of known volume connected by an isolation valve, as shown in Figure 1. The sample for measurement is loaded into the sample volume and the initial pressure reading is taken. For absorption the reservoir is filled with the sorption gas to a predetermined pressure above that of the initial pressure in the sample volume. The isolation valve between the two volumes is opened and the gas is allowed to equilibrate between the reservoir and sample volume. By knowing the initial gas pressures and the volumes of the system, the quantities of absorbed or desorbed gas can be determined.
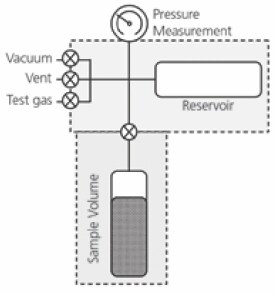
Figure 1.Schematic of a Sieverts Apparatus
By applying the Sievert’s approach to a system using a wide range of calibrated volumes, advanced pressure control, pressure measurements, and temperature control, it is possible to make a full suite of analyses with one device. Such analyses include:
Capacity
The total amount of gas absorbed or desorbed by a sample is pressure and temperature dependent and therefore requires precise measurements of both. In the volumetric method, capacity is directly related to pressure change. Small amounts of impurities in the test gas may be strongly absorbed by the sample. This can be very problematic in gravimetric methods as this uptake can cause significant weight change that might be misinterpreted. In the volumetric method, low levels of impurities may impact the performance of the material, but will not produce significant pressure changes.
Kinetics
Kinetics consists of the dynamic measurement of the change in moles of sorbed gas versus time. An advanced sorption analyzer should consist of multiple reservoirs with a wide range of volumes to match sample size and a wide range of sorption conditions associated with each different type of material. Additionally, sorption reactions generally involve significant endothermic or exothermic reactions. Heat transfer from the sample is a critical aspect of proper kinetics measurements. In a gravimetric instrument the sample is suspended from a microbalance that does not allow direct contact of temperature measuring probes or provide good heat transfer, limiting its usefulness.
Pressure-Composition Isotherms
Pressure-Composition Isotherms (PCT or PCI) is one of the most informative sorption measurements. The result is a plot of the equilibrium absorbed gas concentration in the material as a function of pressure and temperature.
The volumetric approach to PCI measurements consists of adding (or removing) gas to the sample volume in a small dose from one of the calibrated reservoirs and waiting for the resulting gas/solid equilibrium. The PCI curve is produced from the concentration of gas sorbed and the final pressure of each dose in a series of many doses that either increase (absorption) or decrease (desorption) in pressure. This is essentially a gas titration process.
The PCI plot will typically show an equilibrium plateau associated with the co-existence of the remaining unreacted material and the gas-reacted material, thus providing a complete phase diagram. For example, metal hydride materials that reversibly react with hydrogen show a well-defined plateau between the solid solution a-phase, where hydrogen is randomly dissolved within the solid matrix, and the b-phase, where hydrogen is in distinct structural sites and has bonded to the host solid material.3 In addition, precise dosing PCI measurements can provide detailed information on the presence of crystal structure changes, new phases and sorption kinetics as a function of gas concentration.
The PCTPro-2000 makes these measurements easy and has been used extensively in research on complex hydrides such as Ti-catalyzed alanates. Work by researchers at the Max Planck Institute for Coal Research in Germany, investigated the kinetic and thermodynamic effects of varying doping levels of Ti on NaAlH4.4 Figure 2 shows a 160 °C PCT curves for 6 different mole-% doping levels of Ti.
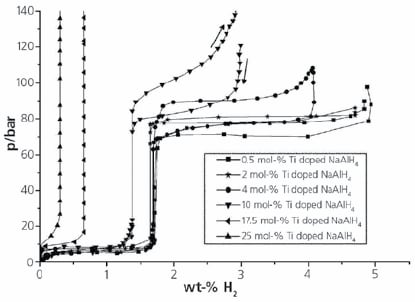
Figure 2.PCT curves for 0.5, 2, 4, 10, 17.5 and 25 mol% Ti doped NaAlH4 at 160 °C. (4)
With the PCTPro-2000, each gas dose can be chosen to be equi-molar and range from extremely small to very large doses (0.1 micro-mole to 10 moles of gas). Now, thanks to the MicroDoser attachment for the PCTPro-2000, it is also possible to extend the full suite of measurement capabilities from very small samples down to milligrams, previously the one real advantage of the gravimetric instruments.
Thermodynamic measurements
The enthalpy and entropy of sorption can be precisely determined by a series of PCI measurements at different temperatures. This results in the van’t Hoff relationship:
ln P = ΔH/RT - ΔS/R
where P is the equilibrium gas pressure, T the absolute temperature, R the gas content, ∆H the reaction enthalpy and ∆S the reaction entropy.5 Figure 3 shows PCT measurements for LaNi5, a classic intermetallic hydride, at six temperatures and the van’t Hoff plot showing the temperature/pressure relationship.6 The PCTPro-2000 instrument enables the direct determination of ΔH and ΔS in a single measurement, thus reducing the measurement time dramatically.
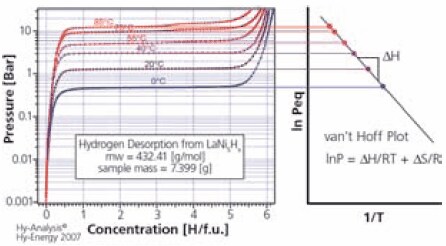
Figure 3.PCT curves and van’t Hoff plot for LaNi5. (3)
Materials Performance Measurements
In addition to the fundamental mechanisms of gas-solid and gas-liquid interactions, Sievert’s instruments are ideal for collecting important material performance data. For example, by making cycle life measurements and catalytic measurements with introduced impurities in the sorption gas, the tolerance of the sample to poisoning can be determined. This information can be very valuable when considering a material’s potential for commercial applications.
Versatility
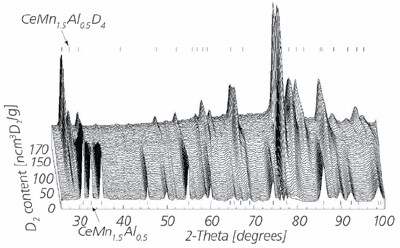
The biggest advantage of the PCTPro-2000 over a gravimetric system is that the sorption measurement is independent of the sample holder design. Unlike gravimetric systems where the sample is restricted to being placed on the balance arm or lever, the sample holder of a volumetric instrument can be of any size, shape, material, position, and location. This makes the volumetric device ideal for simultaneous secondary measurements such as in situ XRD, IR, neutron diffraction, spectrometry, thermal conductivity, electrical conductivity, and many more. An example of the simultaneous controlled deuteration and neutron diffraction of CeMn1.5Al0.5 is shown in Figure 4a and b. The possibilities for in situ measurement of other materials properties are unlimited.
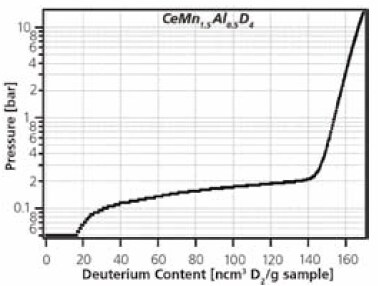
Figure 4b.Deuterium capacity correlated with neutron diffraction patterns.
References
To continue reading please sign in or create an account.
Don't Have An Account?