LC-MS Analysis of Serum for a Wide Analyte Range Using a Novel HLB SPE Phase
M. James Ross, Senior Scientist
Article from Analytix Reporter - Issue 10
Abstract
In this study, a mixture of twenty compounds, the majority pharmaceuticals, with LogP values ranging from -0.89 to 4.65 were analyzed in calf serum, highlighting the broad utility of the new hydrophilic lipophilic balanced (HLB) SPE phase, Supel™ Swift HLB.
Introduction
The preparation of biological samples can have a large impact on the reproducibility and accuracy of their analytical results.1 But, solid phase extraction (SPE) provides an opportunity to reduce matrix effects such as ion suppression and aid in the reliability of consistent results. Hydrophilic-Lipophilic Balanced (HLB) cartridges contain a sorbent offering good wettability for hydrophilic compounds, in addition to providing reverse phase retention.2 These properties allow HLB cartridges to effectively handle a broad range of compounds with varying properties. In this study, a mixture of twenty example compounds with LogP values ranging from -0.89 to 4.65 were analyzed from spiked calf serum using a Supel™ Swift HLB SPE cartridge for cleanup and LC-MS determination.
Experimental Method
A series of twenty analytes and sixteen internal standards as listed in Tables 1& 2 were spiked into calf- serum and allowed to equilibrate for an hour. The calf-serum sample was diluted with an equal volume of 0.4% aqueous formic acid and mixed before the sample was loaded onto the Supel™ Swift HLB SPE cartridge (1 mL/30 mg), and another commercially available HLB cartridge (1 mL/30 mg) for comparison.
Recovery and Ion Suppression/Enhancement
The analytes were monitored at two different transitions of quantifier and qualifier (for confirmation), by a scheduled MRM method. Samples were analyzed against matrix-matched calibration curves. The external standard calibration used six concentrations of the analytes between 20 to 150 ng/mL and a fixed concentration of 50 ng/mL for the internal standards when applicable.
Analytes were spiked in triplicates into calf-serum at concentrations of 100 ng/mL, with a fixed internal standard of concentration 50 ng/mL, before their processing by either the 3-step or 5-step method as shown in Figure 1. The samples were dried and resuspended in starting mobile phase before duplicate analysis by LC-MS/MS on an Agilent 1290 Infinity LC attached to a Sciex 3200 QTRAP® system. The LC conditions including the starting mobile phase are listed in Table 3. A representative trace from recovery is shown in Figure 2.
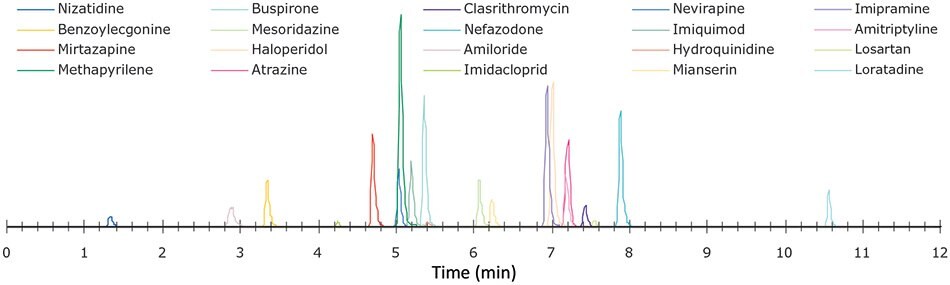
Figure 2.Representative chromatogram of analytes and internal standards for 5-Step method showing individual analytes. For retention times see Table 1.
Results & Discussion
The recoveries of the 20 analytes following their SPE cleanup from both the 3-Step and 5-Step methods of the Supel™ Swift HLB SPE cartridges are presented in Table 4 and Figure 3. Overall, the 5-Step method showed better recovery (100.7 ±6.8%) compared to the 3-Step process (85.1 ±4.2%), partly because of the two earliest eluting compounds (amiloride and nizatidine, both below 70% recovery in 3-Step method). Under the 5-Step process, all twenty analytes had recoveries between 80% and 120%. For 16 of the 20 analytes, the 3-Step process achieved recoveries in the 80% to 120% range.

Figure 3.Summary of recovery for the 3-Step and 5-Step process using Supel™ Swift HLB SPE cartridges. Analytes are in order of increasing LogP values.

Figure 4.Percent recovery of 5-Step method for Supel™ Swift HLB SPE cartridges and a commercially available HLB product. Analytes are arranged in order of increasing LogP values.
In addition to evaluating the performance of the Supel™ Swift HLB SPE cartridges, its performance was also compared with another commercially available HLB cartridge. Both cartridges contained 30 mg of resin and had a max sample volume of 1 mL. To compare the effectiveness of these cartridges, the 5-Step method was employed for the same set of analytes and under similar conditions. Figure 4 and Table 4 represent the recovery using the two cartridges. As previously mentioned, all analytes had a recovery in the range of 80-120% when using the Supel™ Swift HLB SPE cartridges. This is in contrast to the other commercially available cartridge where only 16 of the 20 analytes (80%) were recovered in the 80-120% range.
Beyond recovery rates, another important factor to consider when performing SPE is the impact of residual background on ion suppression and/or ion enhancement. The ion suppression/enhancement is an indication of how well the SPE step removes matrix components (in this case from calf-serum). As displayed in Figure 5, for 16 of the 20 analytes (80%) processed with Supel™ Swift HLB SPE cartridges, there was minimal impact on ionization (≤ +/- 10%). For the remaining 4 analytes, ion enhancement but no ion suppression was observed. This is in stark contrast to the other commercially available cartridge, where 14 of the 20 analytes (70%) showed an ionization suppression (13 of the 14) or enhancement (1 of 14) of more than 10%. To conclude, samples prepared using the Supel™ Swift HLB SPE cartridges showed less ion suppression/enhancement when compared to another commercially available cartridge.

Figure 5.Signal suppression or enhancement effects for Supel™ Swift HLB SPE cartridges and another commercially available HLB product using the 5-Step method. Analytes are arranged in order of increasing LogP values.
Conclusion
Through the use of twenty analytes with various log P values, the Supel™ Swift HLB SPE cartridges demonstrated excellent recoveries (100% of analytes in the range of 80-120% with the 5-step method) and minimal effects on analyte ionization for 80% of the analytes, indicating a good matrix removal.
The advantages of the Supel™ Swift HLB SPE cartridges were further demonstrated on their comparison to a commercially available HLB cartridge under the same set of conditions.
References
To continue reading please sign in or create an account.
Don't Have An Account?