Characterization of Stable Isotope Caffeine as Reference Material for Isotope Dilution Mass Spectrometry
Rudolf Köhling, Christine Hellriegel,
Analytix Volume 2, 2015
Organic TraceCERT® CRMs
Introduction
Isotope dilution mass spectrometry (IDMS) is a precise method to determine concentrations of a given analyte in pure materials or in any type of matrix. This technique is based on the direct proportionality of mass fraction ratio and signal intensity ratio of the natural isotope and an isotopically labeled form of the target analyte. Since the measurement of the signal ratio is directly traceable to an SI unit (mass), this technique is a primary method like qNMR. A closer look at mass spectrometry shows some advantages over NMR or titration, such as higher sensitivity, analysis of matrix reference materials and independence of solvent selection[1–5].
Experimental
Most important for precise IDMS results is the exact determination of the intensity ratio of caffeine and caffeine-13C3. Therefore a large number of single spectra are acquired to minimize the noise caused by the ionization process. Trapping a peak in an injection loop and a slow infusion with a second pump into the ion source allows the acquisition of many more spectra than the direct detection of a chromatographic peak. Peak trapping or direct injection reduces the uncertainty of IDMS data, even of fast scanning mass spectrometers[1–2].
In this particular case, mixtures of labeled and unlabeled caffeine with varying mass fraction ratios were weighed in a sample vial and dissolved in water. Since the labeled caffeine is analyzed as sample, the unlabeled TraceCERT caffeine (56396) serves as reference standard to determine the content of caffeine-13C3 (Product No. 485365). The total mass of the mixtures was ca. 10 mg. All sample solutions were diluted 1:100 and injected into a 10 µL sample loop and introduced into the ion source with a syringe pump. The mobile phase is water/methanol (50:50, v/v). 1H qNMR data of the labeled caffeine were acquired in parallel to the IDMS experiments using maleic acid as reference material. The resulting caffeine content represents the total amount of all caffeine species.
Results
The technique presented reduces the uncertainty contribution of typical noise of ESI data due to the larger number of acquired spectra and the improved statistics of the calculated intensity ratios of 195/198 m/z (caffeine/caffeine-13C3).

Figure 1.The intensity ratio of 195 and 198 m/z is calculated from this spectrum and compared with the average of the intensity ratios of the single mass spectra.
Calibration Curve of the Different Mass Fractions m(Caffeine)/ m(Caffeine-13C3)
Plotting the intensity ratios I(198)/I(195) or I*/I versus the corresponding mass fractions m(caffeine-13C3)/m(caffeine) or m*/m results a straight line (Figure 2) indicating no interferences between labeled and unlabeled caffeine.

Figure 2.Calibration curve calculated from the 6 individual caffeine/caffeine-13C3 mixtures at 3 m*/m ratios (5 mg/15 mg, 10 mg/10 mg, 15 mg/ 5 mg).
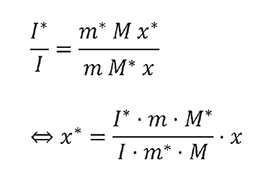
The unknown content of caffeine-13C3 can be calculated according to the above equation [1] for each single sample or from the slope of the calibration curve.
Finally the IDMS experiments result in a content of 98.6% +/- 0.5% caffeine- 13C3. qNMR results in 98.2% +/- 0.3% and the content according to the Certificate of Analysis of Product No. 485365 Lot EB0276V is 98.5% caffeine-13C3.
References
To continue reading please sign in or create an account.
Don't Have An Account?