All Photos(1)
About This Item
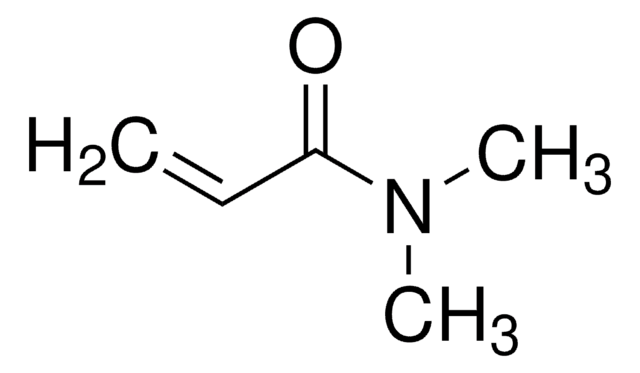
Empirical Formula (Hill Notation):
C9H8N2
CAS Number:
Molecular Weight:
144.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
122-124 °C (lit.)
SMILES string
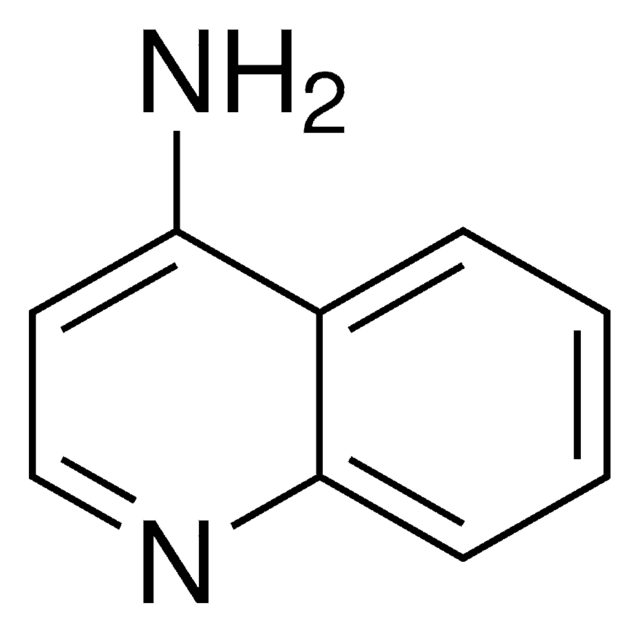
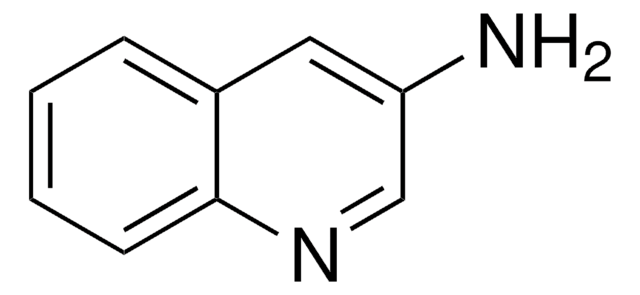
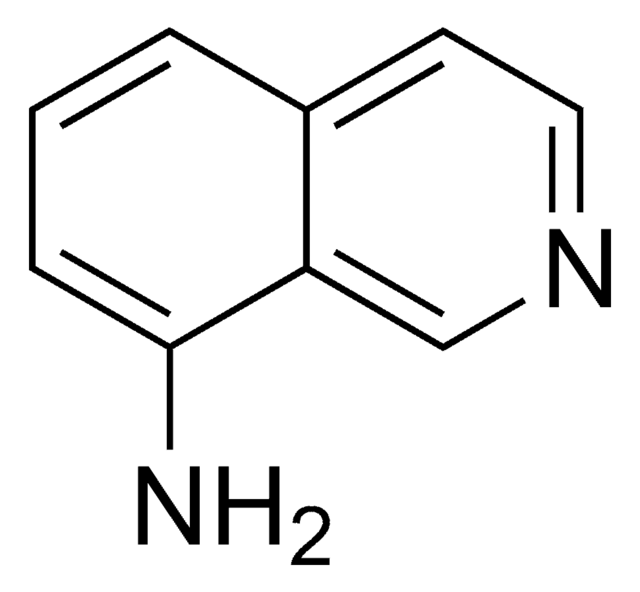
Nc1nccc2ccccc12
InChI
1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11)
InChI key
OSILBMSORKFRTB-UHFFFAOYSA-N
Application
1-Aminoisoquinoline was used in the synthesis of pyrimidoisoquinolinone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Adrian L Smith et al.
Journal of medicinal chemistry, 52(20), 6189-6192 (2009-09-22)
The discovery and optimization of a novel series of aminoisoquinolines as potent, selective, and efficacious inhibitors of the mutant B-Raf pathway is presented. The N-linked pyridylpyrimidine benzamide 2 was identified as a potent, modestly selective inhibitor of the B-Raf enzyme.
Danqing Zheng et al.
Organic & biomolecular chemistry, 9(13), 4763-4765 (2011-05-28)
2-Alkynylbenzaldoxime reacts with amine catalyzed by silver triflate under mild conditions, leading to 1-aminoisoquinolines in good yield. This reaction proceeds efficiently with good functional group tolerance.
Scott P Brown et al.
Journal of medicinal chemistry, 52(10), 3159-3165 (2009-04-24)
We apply a high-throughput formulation of the molecular mechanics with Poisson-Boltzmann surface area (htMM-PBSA) to estimate relative binding potencies on a set of 308 small-molecule ligands in complex with the proteins urokinase, PTP-1B, and Chk-1. We observe statistically significant correlation
Hervé Bibas et al.
The Journal of organic chemistry, 67(8), 2619-2631 (2002-04-13)
The synthesis, spectroscopic properties, and chemical reactions of the stable (neopentylimino)-, (mesitylimino)-, and (o-tert-butylphenylimino)propadienones (6) are reported. Nucleophilic addition of amines affords the malonic amidoamidines 7 and 8. 3,5-Dimethylpyrazole reacts analogously to form 9b. Addition of 1,2-dimethylhydrazine produces pyrazolinones 10-12.
J B Rewinkel et al.
Bioorganic & medicinal chemistry letters, 9(5), 685-690 (1999-04-14)
Replacement of the highly basic benzamidine moiety of NAPAP by the moderately basic 1-aminoisoquinoline moiety resulted in thrombin inhibitors with improved selectivity towards trypsin and enhanced Caco-2 cell permeability.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service