SHP001
MISSION® Lentiviral Packaging Mix
Production of replication incompetent viral particles
Synonym(s):
Lentiviral Packaging, Lentiviral Packaging Kit
About This Item
Recommended Products
Related Categories
General description
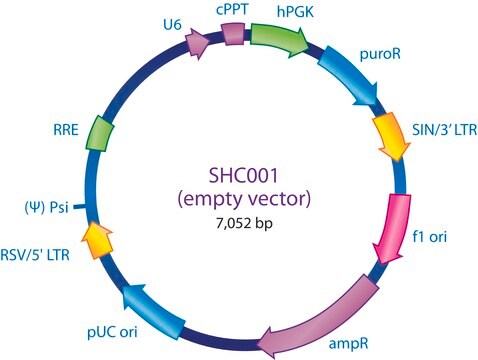
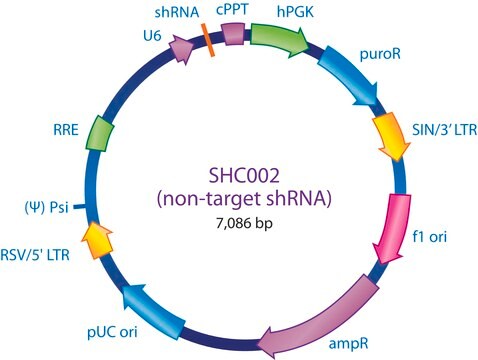
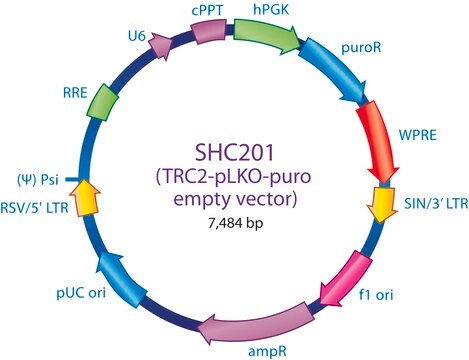
The MISSION® Lentiviral Packaging Mix is an optimized formulation of two plasmids expressing the key HIV packaging genes and a heterologous viral envelope gene.
Lentiviral particles are generated from three main components:
- The packaging vector, which contains the minimal set of lentiviral genes required to generate the virion structural proteins and packaging functions.
- The vesicular stomatitis virus G-protein envelope vector, which provides the heterologous envelope for pseudotyping.
- The shRNA transfer vector, which contains the sequence of interest as well as the cis acting sequences necessary for RNA production and packaging.
The Lentiviral Packaging Mix contains the first two components; it is designed to be co-transfected along with a compatible lentiviral transfer vector in order to create high-titer pseudo-typed lentiviral particles used for downstream transduction applications.
Application
Other Notes
Legal Information
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
MISSION shRNA reduce the expression of specific target genes by targeting the specific mRNA therefore reducing the corresponding protein expression.
Protocols
Preparation of the Lentiviral Transduction Particles Using Packaging Plasmid Mix
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service