Purification or Removal of Biotin and Biotinylated Biomolecules with Magnetic Beads
The magnetic beads for separation of biotinylated biomolecules have a Streptavidin ligand. Streptavidin is an Mr 60 000 protein from Streptomyces avidinii, and is a tetramer containing four biotin binding sites. Since the affinity between streptavidin and biotin is exceptionally high, harsh conditions are required for disruption, such as the use of SDS in the sample buffer. Therefore, it is also possible to use the beads for immunoprecipitation and elute binding partners in an interaction complex without coeluting the biotinylated component. The principle of immunoprecipitation is shown in Figure 1.
Streptavidin Mag Sepharose is used for efficient enrichment of biotinylated proteins, such as antibodies and immunoprecipitation. Streptavidin-coated Sera-Mag and Sera-Mag SpeedBeads are designed for isolation of biotinylated targets such as PCR products, oligos, and antibodies. The beads are generally used for increased throughput and precision in immunoassays. Neutravidin-coated and Streptavidin-blocked beads have reduced nonspecific binding, which can be beneficial in some applications.
Bead characteristics
Characteristics of Mag Sepharose, Sera-Mag, and Sera-Mag SpeedBeads for the capture of biotin and biotinylated substances are shown in Table 1.
| Product | Ligand | Matrix | Binding capacity | Average particle size (µm) |
|---|---|---|---|---|
| Streptavidin Mag Sepharose | Streptavidin | Highly cross-linked agarose with magnetite | 300 µg biotinylated BSA/mL medium slurry | 37 to 100 |
| Sera-Mag Streptavidin-Coated | Streptavidin | Polymer beads with single layer of magnetite | Biotin (pmol/mg): Low 2500 to 3500 Medium 3500 to 4500 High 4500 to 5500 | 1 |
| Sera-Mag SpeedBeads Streptavidin-Coated | Streptavidin | Polymer beads with double layer of magnetite | Biotin (pmol/mg): Low 2500 to 3500 Medium 3500 to 4500 High 4500 to 5500 | 1 |
| Sera-Mag SpeedBeads Streptavidin-Blocked | Streptavidin | Polymer beads with double layer of magnetite | Fluorescein (pmol/mg): Medium ~ 3500 | 1 |
| Sera-Mag SpeedBeads Neutravidin-Coated | Neutravidin | Polymer beads with double layer of magnetite | Biotin (pmol/mg): Medium 3500 to 4500 | 1 |
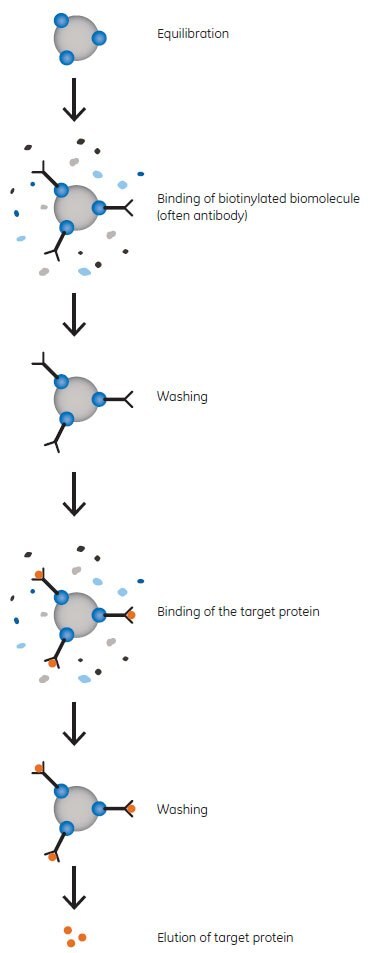
Figure 1. Principle of Immunoprecipitation
Purification Example
Immunoprecipitation of Low Concentration Transferrin from Large Sample Volumes
The ability to use different volumes of sample and medium slurry is one of the key advantages of the magnetic beads separation method. Streptavidin Mag Sepharose was used to purify human transferrin spiked in 5 mg/mL of E. coli lysate. Purification was scaled up 10-fold from 50 to 500 µl of Streptavidin Mag Sepharose and 3 to 30 mL of sample, respectively. The experiment was performed in duplicate using MagRack Maxi. The transferrin concentration was 0.75 µg/mL, which corresponds to ~ 0.015% of the total E. coli protein content. Transferrin was captured by immunoprecipitation using a biotinylated polyclonal rabbit antihuman transferrin immobilized on the medium and the yield of transferrin was estimated by SDS-PAGE of the eluted fractions.
The results (Figure 2) show the inherent flexibility of Streptavidin Mag Sepharose. A corresponding increase in the purity and recovery of transferrin was observed when the immunoprecipitation was scaled up 10-fold. The yields of transferrin were 1.2 and 13 µg using 50 and 500 µl of Streptavidin Mag Sepharose slurry, respectively. The average purity was 78% (5200-fold enrichment) and 75% (5000-fold enrichment).
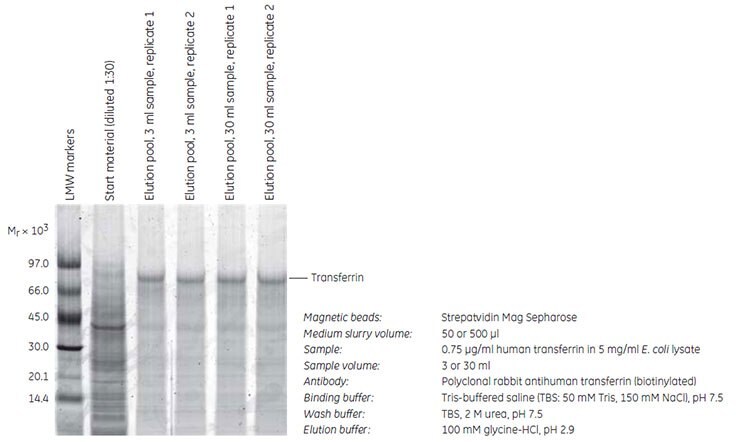
Figure 2. SDS-PAGE (reducing conditions) stained with Deep Purple Total Protein Stain. The purity and recovery obtained were equally high when the scale of purification was increased 10-fold. Quantitation of the eluted transferrin was performed using standard curves with known amounts of transferrin (data not shown).
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?