CRM04291
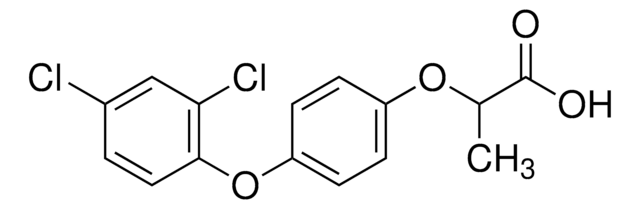
Diclofop-methyl
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
About This Item
Produtos recomendados
grau
certified reference material
TraceCERT®
Nível de qualidade
linha de produto
TraceCERT®
prazo de validade
limited shelf life, expiry date on the label
fabricante/nome comercial
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
cadeia de caracteres SMILES
COC(=O)C(C)Oc1ccc(Oc2ccc(Cl)cc2Cl)cc1
InChI
1S/C16H14Cl2O4/c1-10(16(19)20-2)21-12-4-6-13(7-5-12)22-15-8-3-11(17)9-14(15)18/h3-10H,1-2H3
chave InChI
BACHBFVBHLGWSL-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com
Diclofop-methyl is a post-emergence chiral herbicide that belongs to the class of aryloxyphenoxy propanoate (AOPP) compounds. It prevents the synthesis of fatty acids by inhibiting the activity of acetyl CoA carboxylase (ACCase). Diclofop-methyl is a selective and systemic herbicide used against wild oats, wild millets, and other annual grass weeds in broad-leaf crops, wheat, barley, maize, sorghum, oats, sugar cane, rice, dicotyledonous vegetables, and cotton. It causes an immediate inhibitory effect on the growth of the shoot, intercalary, and root meristems of the plant. It quickly hydrolyzes to give diclofop in plants, water, and soil.
Diclofop-methyl was included on 1st June 2011 in Annex I of Directive 91/414/EEC by the European Commission Directive 2011/45/EU. Diclofop-methyl is approved for use in the European Union under EC Regulation No 1107/2009, as per the Commission Implementing Regulation (EU) No 540/2011, however it is a candidate for substitution.
Aplicação
The diclofop-methyl CRM may find its use as described below:
- To study the enantioselective degradation of diclofop-methyl and diclofop in two soil samples under aerobic and anaerobic conditions using high-performance liquid chromatography (HPLC)
- Simultaneous extraction and determination of enantiomers of diclofop-methyl and diclofop in cole using high-performance liquid chromatography-chiral stationary phase (HPLC-CS)
- Development of a multi-residue method for simultaneous estimation of 16 pesticides in water samples using direct immersion SPME followed by GC-MS analysis
- Simultaneous extraction and determination of aryloxyphenoxy-propionate herbicides from water samples by dispersive magnetic solid-phase extraction (d-MSPE) with high-performance liquid chromatography-diode array detector (HPLC-DAD) and ultra-high pressure liquid chromatography -triple quadrupole mass spectrometer (UHPLC-MS/MS)
- Evaluating the capacity of silica-bonded deoxycholic acid as a stationary phase having a 3,5-dinitrophenylcarbamoyl and a calix[4]arene substituent for enantiomeric separations
Produtos recomendados
Informações legais
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Sens. 1
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica