SRP9000
Prolactin human
human, recombinant, expressed in HEK 293 cells
Sinônimo(s):
PRL
About This Item
Produtos recomendados
fonte biológica
human
Nível de qualidade
recombinante
expressed in HEK 293 cells
esterilidade
non-sterile
Ensaio
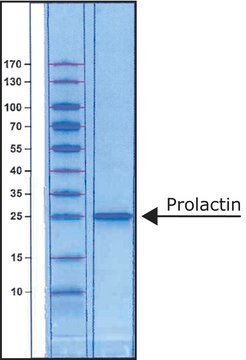
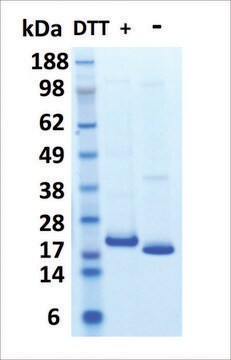
≥95% (SDS-PAGE)
forma
liquid
potência
≤2 ng/mL Nb2-11 cells proliferation EC50
prazo de validade
2 yr
peso molecular
23 kDa
técnica(s)
cell culture | mammalian: suitable
Impurezas
≤1 EU/μg protein Endotoxin level
temperatura de armazenamento
−20°C
Informações sobre genes
human ... prl(5617)
Descrição geral
Prolactin (PRL) is a multifunctional polypeptide hormone primarily produced by the lactotrophic cells of the anterior pituitary gland in vertebrates.
Aplicação
- in in vitro experiments to examine its effects in sleep-like concentrations on T-cell migration
- to study its effects on claudin 2 (CLDN2) expression in the Caco-2 intestinal epithelial cell model
- in microplate assays to demonstrate the specificity of the antibodies for vasoinhibin
Ações bioquímicas/fisiológicas
forma física
Nota de preparo
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Repr. 1B
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica