MTOX1016
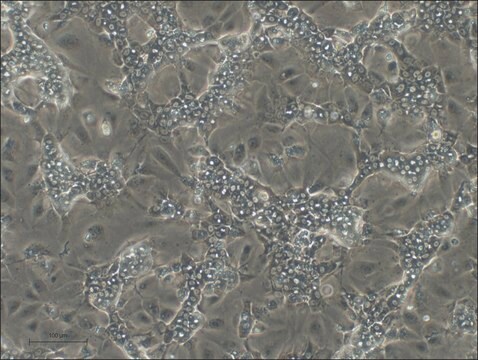
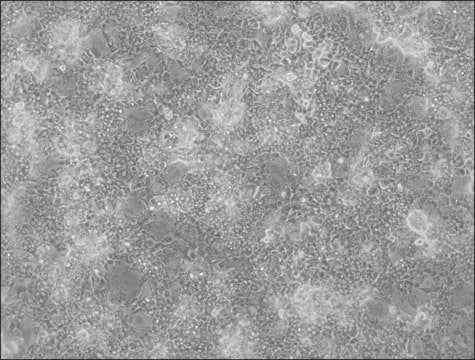
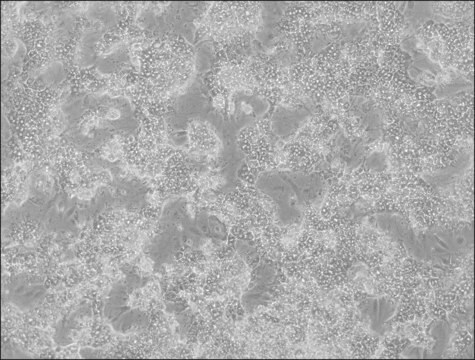
OATP1B3 Knockout HepaRG™ Cells
human female liver (hepatocarcinoma and hepatitis C tumor)
About This Item
Produtos recomendados
Nome do produto
OATP1B3 Knockout HepaRG™ Cells, 1 vial
fonte biológica
human female liver (hepatocarcinoma and hepatitis C tumor)
Nível de qualidade
Formulário
liquid
nº de adesão OMIM
temperatura de armazenamento
−196°C
Informações sobre genes
human ... OATP1B3(28234)
Descrição geral
Aplicação
Características e benefícios
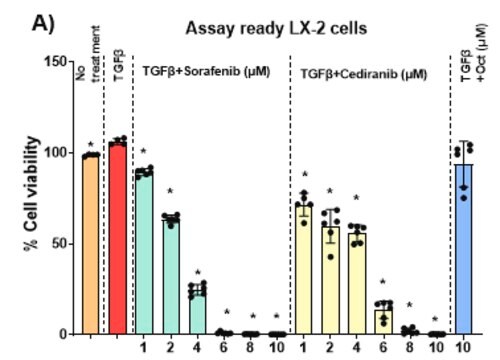
- The frame-shift mutation of SLCO1B3 gene was confirmed by fragment length analysis and DNA sequencing.
- Loss of functionality was confirmed by loss of transport of selective substrates in sandwich culture assay.
Qualidade
Informações legais
Exoneração de responsabilidade
Código de classe de armazenamento
12 - Non Combustible Liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
Oral drug delivery involves dissolution in the small intestine and absorption across the enterocyte barrier into the portal vein followed by subsequent delivery through the liver into the systemic circulation.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica