92549
Acenaphthylene
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
About This Item
Produtos recomendados
grau
certified reference material
TraceCERT®
Nível de qualidade
linha de produto
TraceCERT®
prazo de validade
limited shelf life, expiry date on the label
fabricante/nome comercial
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
técnica(s)
HPLC: suitable
gas chromatography (GC): suitable
pb
280 °C (lit.)
pf
78-82 °C (lit.)
89-92 °C
densidade
0.899 g/mL at 25 °C (lit.)
aplicação(ões)
environmental
formato
neat
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
c1cc2C=Cc3cccc(c1)c23
InChI
1S/C12H8/c1-3-9-4-2-6-11-8-7-10(5-1)12(9)11/h1-8H
chave InChI
HXGDTGSAIMULJN-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com.
Aplicação
Embalagem
Informações legais
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
251.6 °F - closed cup
Ponto de fulgor (°C)
122.0 °C - closed cup
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Protocolos
GC Analysis of PAHs on SLB®-5ms
US EPA Method 610 describes the analysis of polynuclear aromatic hydrocarbons (commonly referred to as PAHs or PNAs) by both HPLC and GC.
GC Analysis of Polynuclear Aromatic Hydrocarbons (PAHs) in Salmon on SPB®-608 (20 m x 0.18 mm I.D., 0.18 µm) after QuEChERS Cleanup using Supel™ QuE Z-Sep, Fast GC Analysis
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica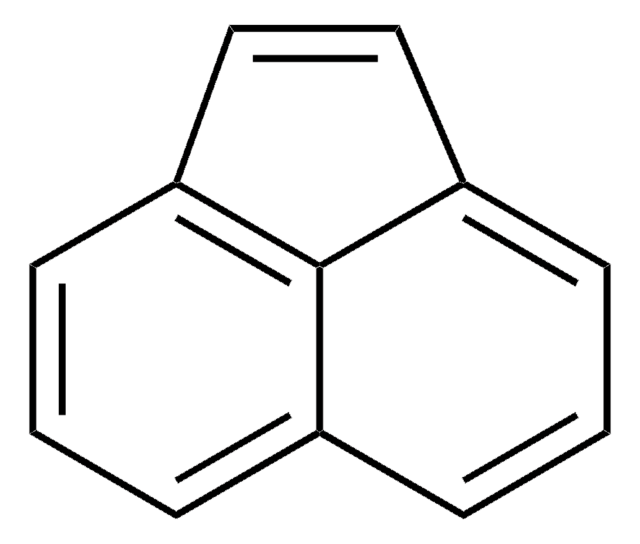



![Benzo[ghi]perylene analytical standard](/deepweb/assets/sigmaaldrich/product/structures/154/740/c50ff1be-dfb4-4159-a98c-9cecf9206ad3/640/c50ff1be-dfb4-4159-a98c-9cecf9206ad3.png)
