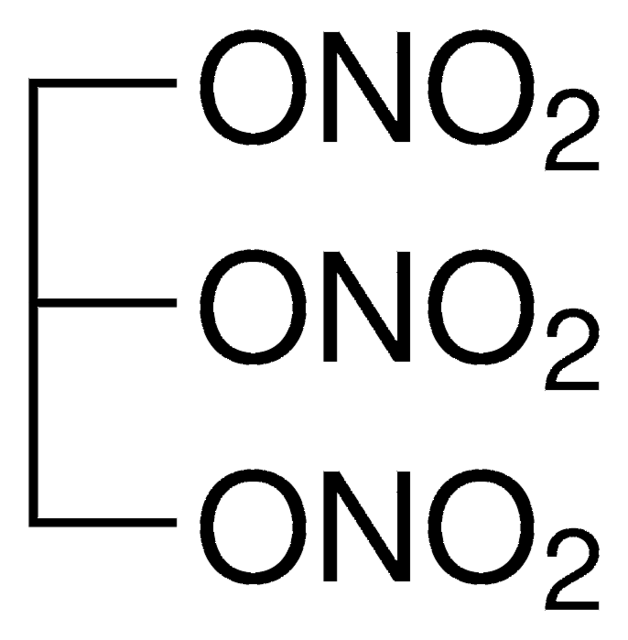
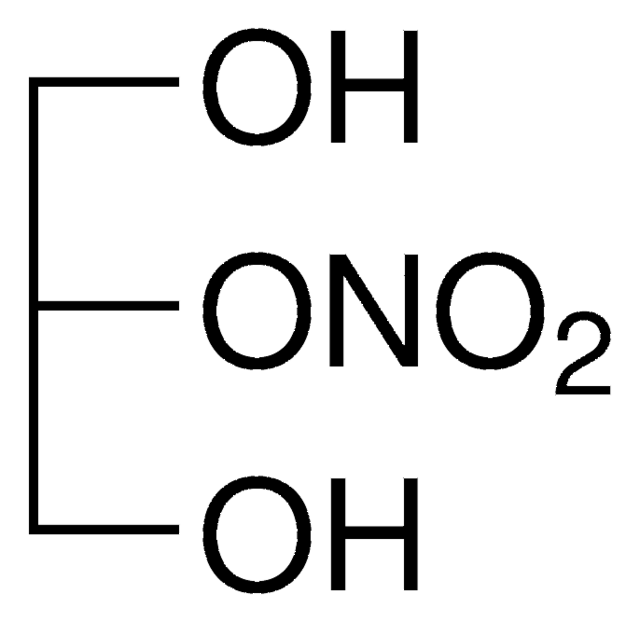
D-011
1,3-Dinitroglycerin solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Produtos recomendados
grau
certified reference material
Nível de qualidade
forma
liquid
Características
Snap-N-Spike®/Snap-N-Shoot®
embalagem
ampule of 1 mL
fabricante/nome comercial
Cerilliant®
concentração
1.0 mg/mL in acetonitrile
técnica(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
aplicação(ões)
pharmaceutical (small molecule)
formato
single component solution
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
OC(CO[N+]([O-])=O)CO[N+]([O-])=O
InChI
1S/C3H6N2O7/c6-3(1-11-4(7)8)2-12-5(9)10/h3,6H,1-2H2
chave InChI
ASIGVDLTBLZXNC-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- Population pharmacokinetics of nitroglycerin and of its two metabolites after a single 24-hour application of a nitroglycerin transdermal matrix delivery system: This study presents a comprehensive analysis on the population pharmacokinetics of nitroglycerin, focusing on its behavior and the behavior of its metabolites following the use of a transdermal delivery system. The findings contribute to understanding the systemic availability and action of nitroglycerin from such systems, which could be relevant for 1,3-Dinitroglycerin given its structural and functional similarities (Auclair et al., 1998).
- Novel pharmacokinetic modelling of transdermal nitroglycerin: This research explores advanced pharmacokinetic models that describe the absorption and action of nitroglycerin when administered through transdermal patches. Such models are crucial for developing effective transdermal delivery systems for similar compounds like 1,3-Dinitroglycerin (Auclair et al., 1998).
- Bioequivalence Comparison of Two Drug-in-Adhesive Transdermal Nitroglycerin Patches: This study compares the bioequivalence of two transdermal patches delivering nitroglycerin, highlighting the critical factors in the design and performance of transdermal systems that could similarly apply to 1,3-Dinitroglycerin (Harrison et al., 1996).
- Percutaneous absorption of 1,3-dinitroglycerin and a trial of pharmacokinetic analysis: Directly relevant to 1,3-Dinitroglycerin, this study examines its percutaneous absorption characteristics, offering insights into its potential applications in transdermal delivery systems (Ogiso et al., 1990).
- Analysis of solutions containing glutathione and inorganic nitrite: application to nitroglycerin metabolism studies: While primarily focused on nitroglycerin, this analysis could provide secondary insights into the metabolic pathways and interactions of 1,3-Dinitroglycerin with biomolecules like glutathione, which is crucial for understanding its pharmacological effects (Curry et al., 1987).
Informações legais
produto relacionado
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
35.6 °F - closed cup
Ponto de fulgor (°C)
2 °C - closed cup
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica