Heterocycle and Cyclic Peptide Formation with N-(isocyamino)triphenylphosphorane (Pinc)
Isocyanides are widely used reagents in organic synthesis, with applications ranging from materials science to drug discovery. In the course of a chemical transformation, the terminal carbon of an isocyanide establishes a connection with both a nucleophile and an electrophile. This is why isocyanides readily participate in multicomponent reactions. Because of the possibility to intercept isocyanide-derived reactive intermediates, additional nucleophilic and electrophilic functionalities in the vicinity of the isocyanide group can lead to the discovery of new reactions. N-(Isocyamino)phosphoranes exemplify such reagents because the nucleophilic amide is sandwiched between positively charged phosphorous atom and amphoteric isocyanide carbon.
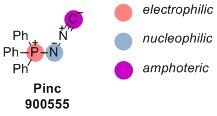
Originally developed by Fehlhammer and co-workers as a precursor to isodiazomethane-transition metal complexes,1 Pinc (Product No. 900555) is a useful reagent for heterocycle synthesis. Ramazani and co-workers reported the reaction of this phosphorane with amine, aldehyde, and carboxylic acid in a reaction analogous to the 4-component Ugi reaction.2 In this process, the key mixed anhydride characteristic of the Ugi reaction is intercepted by the amide nitrogen of Pinc. Loss of triphenylphosphine oxide affords disubstituted 1,3,4-oxadiazoles.

Yudin and coworkers have recently demonstrated the utility of Pinc for the macrocyclization of peptides.3 An electrostatic interaction between the two termini of the pre-cyclization intermediate is believed to promote efficient ring closure. The product macrocycles contain the oxadiazole incorporated into the backbone, which acts as endocyclic conformational control element, locking macrocyles into single conformations in solution, which can allow for optimization of their biological properties. Importantly, the resulting peptidomimetic macrocycles exhibit improved membrane permeability, lipophilicity, and aqueous solubility.3
Advantages of Pinc:
- Bench-stable solid
- Simultaneously facilitates cyclization and incorporation of conformational control element into peptide macrocycles
- Amphoteric properties can allow for the design of new multicomponent reactions
- Cell-permeability and rigid conformation of resulting cycle peptides are desired drug properties
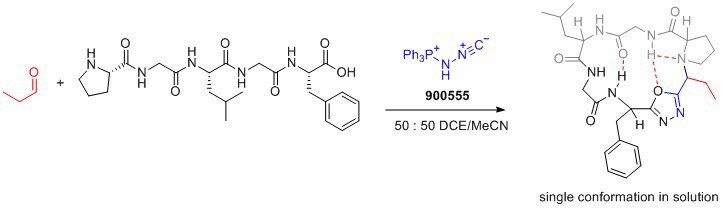
The capability to form oxadiazoles using Pinc and the unique reactivity of this bench-stable reagent opens up the door for the development of new transformations.
References
To continue reading please sign in or create an account.
Don't Have An Account?