General description
Lot specific orders are not able to be placed through the web. Contact your local sales rep for more details.
Application
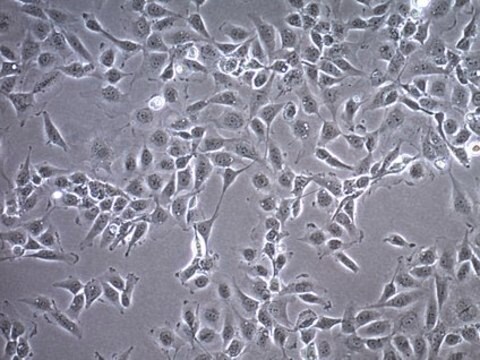
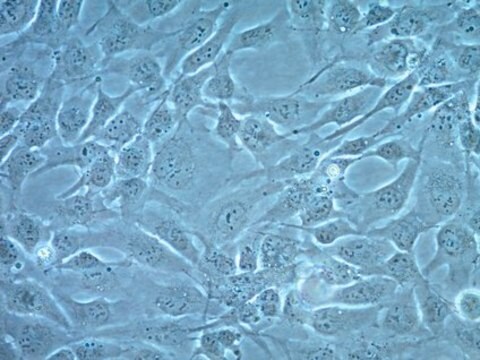
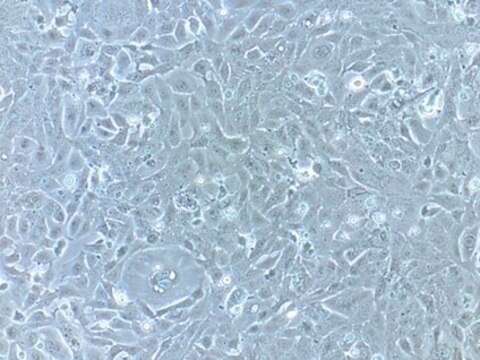
Primary Human Cardiac Myocytes (HCM) are isolated from the ventricles of the adult heart. They are qualified for in vitro research on cardiac diseases and for pharmacological studies. Unlike freshly isolated rod-shaped myocytes, cultured HCM can be used for long-term experiments like investigating the long-term effects of cytokines, mechanical strain, or cell-cell interactions, as they are prepared according to a special protocol.Initially, the HCM act more like progenitor cells in that they are not yet fully differentiated. They express the markers of early stage differentiation such as GATA-4 and sarcomeric alpha-actin and have a high capacity for proliferation. When they are grown to confluency and cultivated for an extended period of time, the differentiation process begins. Markers of late differentiation (e.g. sarcomeric alpha-actinin, slow muscle myosin) are increased and the cells begin to form myotube-like structures.
Quality
Rigid quality control tests are performed for each lot of the corresponding cells. They are tested for cell viability, cell morphology, growth performance and for cell-type specific markers. In addition, all cells have been tested for the absence of HIV-1, HIV-2, HBV, HCV, HTLV-1, HTLV-2 and microbial contaminants (fungi, bacteria, and mycoplasma).
Warning
Although tested negative for HIV-1, HIV-2, HBV, HCV, HTLV-1 and HTLV-2, the cells – like all products of human origin – should be handled as potentially infectious. No test procedure can completely guarantee the absence of infectious agents.
Physical form
Pellet is dissolved in RNAlater® for downstream analysis of DNA, RNA or protein profiles. Cells are not revivable.
Subculture Routine
Click
here for more information.
Other Notes
The Cell Pellets can be stored indefinitely at -20°C, and for up to 1 month at 4°C. Note: The Cell Pellets will not freeze at -20°C. Always centrifuge product briefly before opening vial.
Legal Information
RNAlater is a registered trademark of Ambion, Inc.
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.