411205
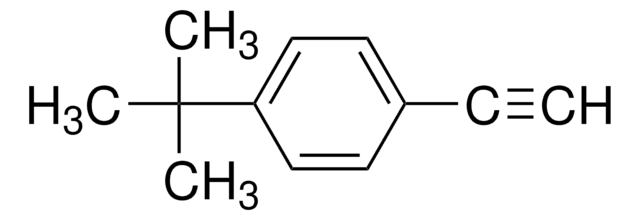
4-tert-Butyliodobenzene
98%
Synonym(s):
1-tert-Butyl-4-iodobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CC6H4I
CAS Number:
Molecular Weight:
260.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.57 (lit.)
bp
116-118 °C/9 mmHg (lit.)
density
1.468 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)c1ccc(I)cc1
InChI
1S/C10H13I/c1-10(2,3)8-4-6-9(11)7-5-8/h4-7H,1-3H3
InChI key
WQVIVQDHNKQWTM-UHFFFAOYSA-N
Related Categories
General description
4-tert-Butyliodobenzene is an electron-rich aryl iodide. Heck reaction between 2-methylprop-2-en-1-ol and 4-tert-butyliodobenzene catalyzed by ionic liquids has been studied. It participates in the one-pot Heck-reductive amination reaction pathway during the synthesis of fungicide fenpropimorph.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Functionalised ionic liquids: synthesis of ionic liquids with tethered basic groups and their use in Heck and Knoevenagel reactions.
Forsyth SA, et al.
New. J. Chem., 34(4), 723-731 (2010)
One-pot multistep synthetic strategies for the production of fenpropimorph using an ionic liquid solvent.
Forsyth SA, et al.
Organic Process Research & Development, 10(1), 94-102 (2006)
Jungwoon Kim et al.
Molecules (Basel, Switzerland), 23(4) (2018-04-13)
Solution-processed organic light-emitting diodes (OLEDs) are attractive due to their low-cost, large area displays, and lighting features. Small molecules as well as polymers can be used as host materials within the solution-processed emitting layer. Herein, we report two 3,3'-bicarbazole-based host
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service