M9411
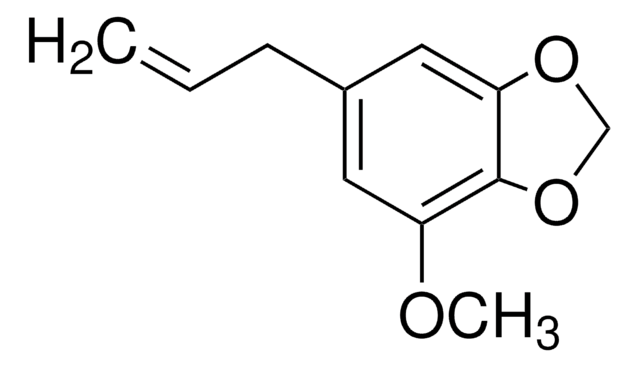
Myristicin from parsley leaf oil
≥85% (HPLC), oil
Synonyme(s) :
4-Methoxy-6-(2-propenyl)-1,3-benzodioxole
Sélectionner une taille de conditionnement
Sélectionner une taille de conditionnement
About This Item
Produits recommandés
Niveau de qualité
Essai
≥85% (HPLC)
Forme
oil
Couleur
clear light yellow
Application(s)
metabolomics
vitamins, nutraceuticals, and natural products
Température de stockage
2-8°C
Chaîne SMILES
COc1cc(CC=C)cc2OCOc12
InChI
1S/C11H12O3/c1-3-4-8-5-9(12-2)11-10(6-8)13-7-14-11/h3,5-6H,1,4,7H2,2H3
Clé InChI
BNWJOHGLIBDBOB-UHFFFAOYSA-N
Catégories apparentées
Description générale
Application
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
STOT SE 3
Organes cibles
Central nervous system
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique