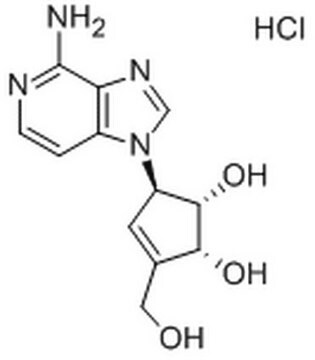
C9492
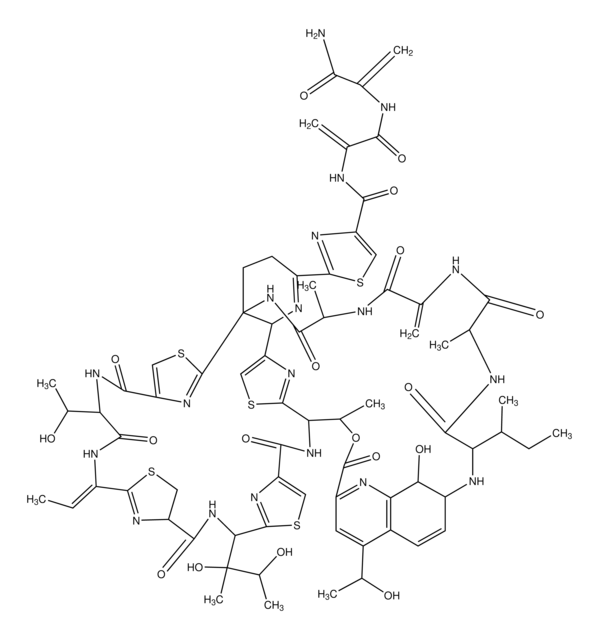
Chaetocin from Chaetomium minutum
≥95% (HPLC)
Synonyme(s) :
(+)-Chaetocin A
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥95% (HPLC)
Forme
powder
Température de stockage
2-8°C
Chaîne SMILES
CN1C(=O)C23CC4(C(Nc5ccccc45)N2C(=O)C1(CO)SS3)C67CC89SSC(CO)(N(C)C8=O)C(=O)N9C6Nc%10ccccc7%10
InChI
1S/C30H28N6O6S4/c1-33-21(39)27-11-25(15-7-3-5-9-17(15)31-19(25)35(27)23(41)29(33,13-37)45-43-27)26-12-28-22(40)34(2)30(14-38,46-44-28)24(42)36(28)20(26)32-18-10-6-4-8-16(18)26/h3-10,19-20,31-32,37-38H,11-14H2,1-2H3
Clé InChI
PZPPOCZWRGNKIR-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Actions biochimiques/physiologiques
Notes préparatoires
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Epigenetic modifications are thought to occur through two key interconnected processes—DNA methylation and the covalent modification of histones.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique