03880590
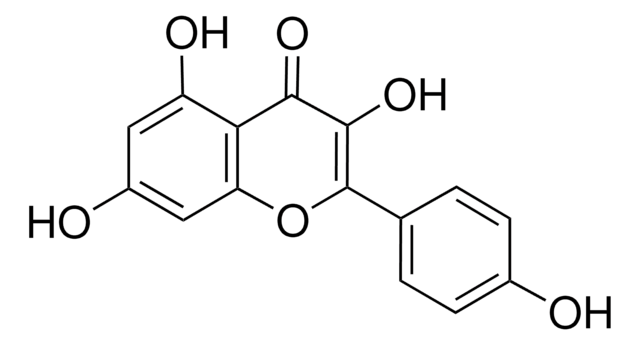
Luteolin
primary reference standard
Synonyme(s) :
3′,4′,5,7-Tetrahydroxyflavone
About This Item
Qualité
primary reference standard
Durée de conservation
limited shelf life, expiry date on the label
Fabricant/nom de marque
HWI
Pf
~330 °C (lit.)
Application(s)
food and beverages
Chaîne SMILES
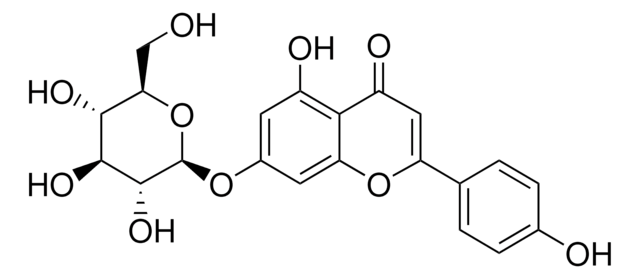
Oc1cc(O)c2C(=O)C=C(Oc2c1)c3ccc(O)c(O)c3
InChI
1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H
Clé InChI
IQPNAANSBPBGFQ-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Exact content by quantitative NMR can be found on the certificate.
Application
Autres remarques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Choose from one of the most recent versions:
Certificats d'analyse (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique