E24905
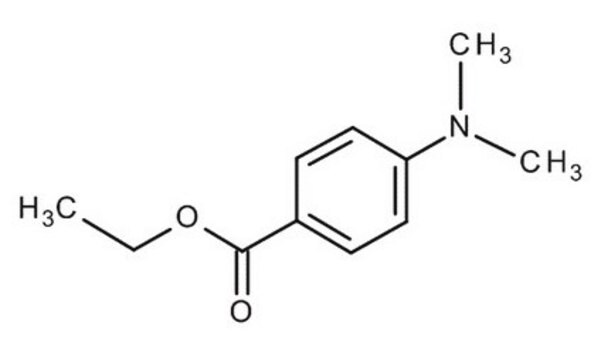
Ethyl 4-(dimethylamino)benzoate
≥99%
Synonym(s):
Parbenate
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
(CH3)2NC6H4CO2C2H5
CAS Number:
Molecular Weight:
193.24
Beilstein:
2210233
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
≥99%
mp
63-66 °C (lit.)
SMILES string
CCOC(=O)c1ccc(cc1)N(C)C
InChI
1S/C11H15NO2/c1-4-14-11(13)9-5-7-10(8-6-9)12(2)3/h5-8H,4H2,1-3H3
InChI key
FZUGPQWGEGAKET-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ethyl 4-(dimethylamino)benzoate (Et-PABA) is a hydrophilic polymer, which can also be used as a derivate of 4-aminobenzoate. It is majorly used as a photo-initiator with a strong estrogenic activity, which finds application as an ultraviolet filter in sunscreens.
Ethyl 4-(dimethylamino)benzoate is a photoinitiator in visible light system.
Application
Can be used as a photoinititator in cell encapsulation applications.
Et-PABA is used as a co-initiator with camphorquinone (CQ) to form a self-adhesive composite for the fabrication of photosensitizers in dental materials.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Effect of photoinitiator combinations on hardness, depth of cure, and color of model resin composites.
Salgado VE, et al.
Journal of Esthetic and Restorative Dentistry : Official Publication of the American Academy of Esthetic Dentistry ... [Et al.], 27, S41-S48 (2015)
Fabrício Aulo Ogliari et al.
Journal of dentistry, 35(7), 583-587 (2007-06-02)
The aim of this study was to evaluate the influence of an onium salt in the polymerization kinetics of a dental adhesive model resin. A monomer mixture, based on Bis-GMA, TEGDMA and HEMA, was used as a model dental adhesive
H Lygre et al.
European journal of oral sciences, 107(5), 378-383 (1999-10-09)
Results are reported from a study on the in vitro separation and identification of leachables from three different polymer-based dental filling materials by using a combined method of gas chromatography and mass spectrometry. The median number of separable organic leachables
Mario Seiss et al.
Archives of toxicology, 83(12), 1109-1115 (2009-09-23)
This study investigated the leaching of ingredients from several commercial dental composite resins cured with LED, and immersed in methanol or water for 24 h, respectively. The composites used were: Admira Dentin (VOCO), Artemis Schmelz (Enamel) (Ivoclar Vivadent), Els extra
Mihaela Roxana Cimpan et al.
Clinical oral investigations, 9(3), 168-172 (2005-05-06)
4-N,N-Dimethyl amino benzoic acid ethylester (DMABEE), a leachable lipophilic component of polymer-based dental-filling materials, has been shown to interact with cell membrane phospholipids, such as phosphatidylcholine and phosphatidylserine (PS). One marker of cellular death by apoptosis is the change in
Articles
The Progress in Development of Dental Restorative Materials
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service