All Photos(1)
About This Item
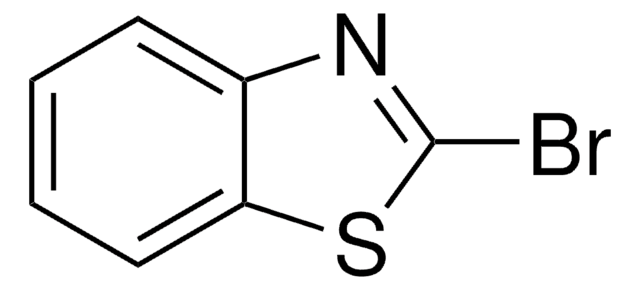
Empirical Formula (Hill Notation):
C3H2BrNS
CAS Number:
Molecular Weight:
164.02
Beilstein:
105724
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.593 (lit.)
bp
171 °C (lit.)
density
1.82 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
Brc1nccs1
InChI
1S/C3H2BrNS/c4-3-5-1-2-6-3/h1-2H
InChI key
RXNZFHIEDZEUQM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Bromothiazole was used to N-arylate 5- and 7-azaindoles. 2-Bromothiazole was also used as starting reagent in the synthesis of:
- 2-cyanothiazole via cpper-catalyzed cyanation
- 2,4,5-trisubstituted thiazoles
- novel electron-deficient fused pyrrolo[3,2-d:4,5-d′]bisthiazole
- 3-(2′-thiazoyl)indoles
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
152.6 °F - closed cup
Flash Point(C)
67 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cora Dunst et al.
The Journal of organic chemistry, 76(16), 6972-6978 (2011-07-09)
A general method for the synthesis of 2,4,5-trisubstituted thiazoles has been developed. Starting from commercially available 2-bromothiazole, successive metalations using TMPMgCl·LiCl or TMP(2)Zn·2MgCl(2)·2LiCl lead to the corresponding magnesated or zincated thiazoles which readily react with various electrophiles providing highly functionalized
Tetrahedron Letters, 48, 4831-4831 (2007)
Synlett, 555-555 (2007)
Mohammed Al-Hashimi et al.
Organic letters, 12(23), 5478-5481 (2010-11-12)
The synthesis of a novel electron-deficient fused pyrrolo[3,2-d:4,5-d']bisthiazole is reported from 2-bromothiazole. This was copolymerized with thiophene, selenophene, thienothiophene, and bithiophene by microwave-assisted Stille polycondensation. The resulting polymers exhibited small optical band gaps combined with low-lying HOMO energy levels and
Synthesis of camalexin and related phytoalexins.
Ayer WA, et al.
Tetrahedron, 48(14), 2919-2924 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)