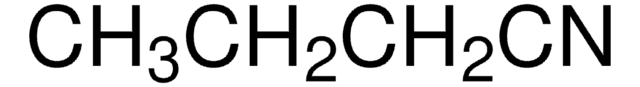Select a Size
All Photos(1)
Select a Size
Change View
About This Item
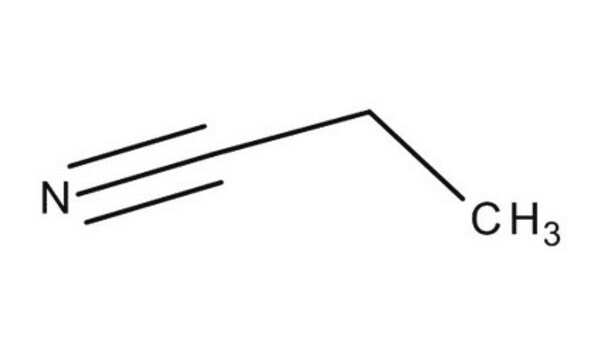
Linear Formula:
CH3CH2CN
CAS Number:
Molecular Weight:
55.08
Beilstein:
773680
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.366 (lit.)
bp
97 °C (lit.)
mp
−93 °C (lit.)
solubility
water: soluble 11.9g/100g at 40 °C
water: soluble 29g/100g at 100 °C
DMF: miscible
alcohol: miscible
diethyl ether: miscible
density
0.772 g/mL at 25 °C (lit.)
functional group
nitrile
SMILES string
CCC#N
Looking for similar products? Visit Product Comparison Guide
General description
Propionitrile is an experimental duodenal ulcerogen and was found to stimulate gastric acid secretion in the rat.
Application
Propionitrile was used in mesoporous graphitic C3N4 catalyzed cyclotrimerisation of various nitriles into triazine derivatives. It was used in Raman spectroscopic study of complex formation between o-cresol and propionitrile.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 2 Oral - Acute Tox. 4 Inhalation - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
42.8 °F - closed cup
Flash Point(C)
6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Pathogenesis of duodenal ulcer. Gastric hyperacidity caused by propionitrile and cysteamine in rats.
S Szabo et al.
Research communications in chemical pathology and pharmacology, 16(2), 311-323 (1977-02-01)
Cysteamine and propionitrile, experimental duodenal ulcerogens, stimulated gastric acid secretion in the rat. Gastric acid secretion was measured by two separate methods, the conventional pylorus ligation technique and a non-invasive technique based on the pH dependent liberation of azure A
A Raman spectroscopic study of complex formation between o-cresol and propionitrile.
Girling RB and Shurvell HF.
Vibrational Spectroscopy, 18(1), 77-82 (1998)
Mesoporous graphitic carbon nitride as a versatile, metal-free catalyst for the cyclisation of functional nitriles and alkynes.
Goettmann F, et al.
New. J. Chem., 31(8), 1455-1460 (2007)
Oliver Kaumanns et al.
The Journal of organic chemistry, 74(1), 75-81 (2008-11-27)
The rates of the reactions of the colored para-substituted phenylacetonitrile anions 1a-c and the phenylpropionitrile anions 2a-c with Michael acceptors (3a-u) were determined by UV-vis spectroscopy in DMSO at 20 degrees C. The reactions follow second-order kinetics, and the corresponding
Atsushi Kunishita et al.
Inorganic chemistry, 47(18), 8222-8232 (2008-08-14)
The copper(II) complexes 1(H) and 1(Ar(X)), supported by the N,N-di(2-pyridylmethyl)benzylamine tridentate ligand (L(H)) or its derivatives having m-substituted phenyl group at the 6-position of pyridine donor groups (L(Ar(X))), have been prepared, and their reactivity toward H2O2 has been examined in
Chromatograms
application for HPLCapplication for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service