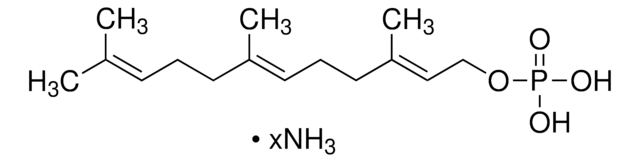
12436
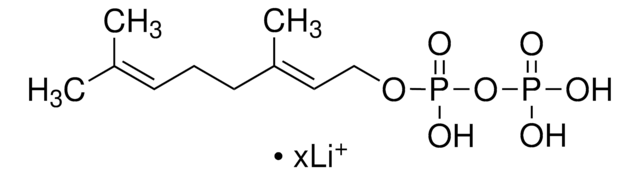
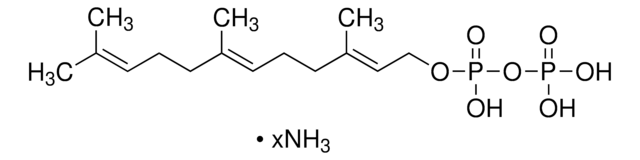
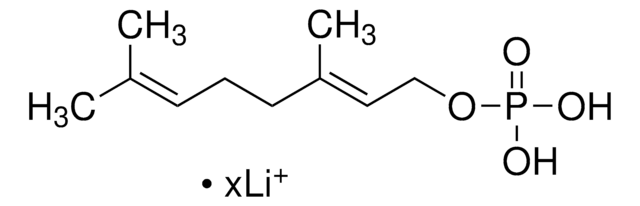
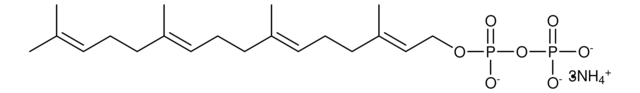
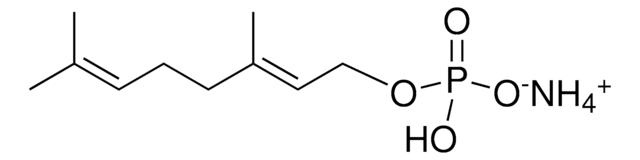
Neryl pyrophosphate lithium salt
≥95.0% (TLC)
Synonym(s):
(Z)-3,7-Dimethyl-2,6-octadien-1-yl pyrophosphate lithium salt, Neryl diphosphate lithium salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H20O7P2 · xLi+
CAS Number:
Molecular Weight:
314.21 (free acid basis)
UNSPSC Code:
12352107
NACRES:
NA.25
Recommended Products
Assay
≥95.0% (TLC)
storage temp.
−20°C
Application
Neryl pyrophosphate, the cis isomer of geranyl pyrophosphate, may be used to characterize and study the kinetics of enzymes such as 1,8-cineole synthase, farnesyl pyrophosphate synthase, pinene cyclase and geranyl pyrophosphate:sabinene hydrate cyclase.
Biochem/physiol Actions
Metabolite, substrate for monoterpene synthase.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R Croteau et al.
Archives of biochemistry and biophysics, 309(1), 184-192 (1994-02-15)
Geranyl pyrophosphate: 1,8-cineole cyclase (cineole synthase) catalyzes the conversion of geranyl pyrophosphate to the symmetrical monoterpene ether 1,8-cineole (1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane) by a process thought to involve the initial isomerization of the substrate to the tertiary allylic isomer, linalyl pyrophosphate, and cyclization
R Croteau et al.
The Journal of biological chemistry, 264(26), 15309-15315 (1989-09-15)
(+)-Pinene cyclase from sage (Salvia officinalis) catalyzes the isomerization and cyclization of geranyl pyrophosphate to (+)-alpha-pinene and (+)-camphene, and to lesser amounts of (+)-limonene, myrcene, and terpinolene, whereas (-)-pinene cyclase from this tissue catalyzes the conversion of the acyclic precursor
D R Light et al.
The Journal of biological chemistry, 264(31), 18598-18607 (1989-11-05)
A prenyltransferase purified from the commercial rubber tree, Hevea brasiliensis, that elongates existing cis-polyisoprene rubber molecules also catalyzes the formation of all trans-farnesyl pyrophosphate (t,t-FPP) from dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP). In assays of the latter activity trans-geranyl
T W Hallahan et al.
Archives of biochemistry and biophysics, 264(2), 618-631 (1988-08-01)
A soluble enzyme preparation from the leaves of sweet marjoram (Majorana hortensis Moench) catalyzes the divalent cation-dependent cyclization of [1-3H]geranyl pyrophosphate to the bicyclic monoterpene alcohols (+)-[6-3H]cis- and (+)-[6-3H]-transsabinene hydrate, providing labeling patterns consistent with current mechanistic considerations. No free
Old substrates for new enzymes of terpenoid biosynthesis.
Jörg Bohlmann et al.
Proceedings of the National Academy of Sciences of the United States of America, 106(26), 10402-10403 (2009-06-26)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service