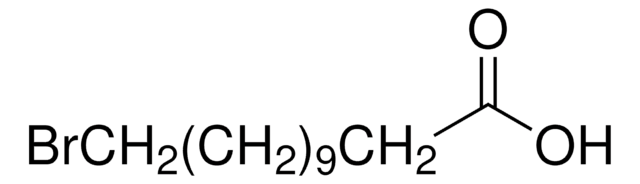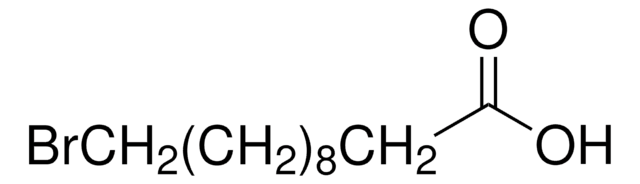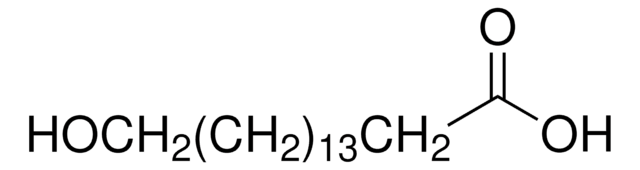All Photos(1)
About This Item
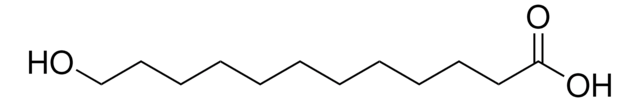
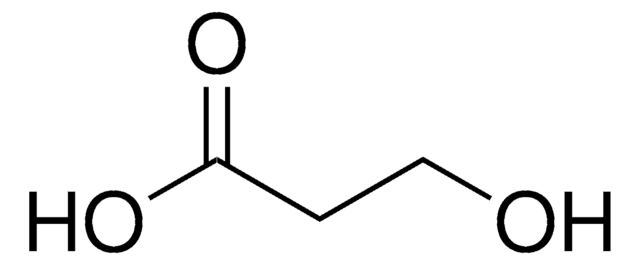
Linear Formula:
HO(CH2)10CO2H
CAS Number:
Molecular Weight:
202.29
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
SMILES string
OCCCCCCCCCCC(O)=O
InChI
1S/C11H22O3/c12-10-8-6-4-2-1-3-5-7-9-11(13)14/h12H,1-10H2,(H,13,14)
InChI key
KNRCBASNXNXUQQ-UHFFFAOYSA-N
Application
11-Hydroxyundecanoic acid can be prepared by employing the following starting reagents:
- 10-undecenoic acid
- undecylenic acid and hydrobromic acid
- methyl 11-bromoundecanoate
- ricinoleic acid (12-hydroxyoleic acid)
11-Hydroxyundecanoic acid may be employed for the preparation of higher molecular weight polyesters.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enzyme-catalysed condensation polymerization of 11-hydroxyundecanoic acid with lipase from Candida cylindracea.
O'Hagan D and Zaidi NA.
Polymer, 35(14), 3576-3578 (1994)
Bis-3-methyl-2-butylborane as a selective reagent for the reduction of representative functional groups.
Brown HC and Bigley DB.
Journal of the American Chemical Society, 83(2), 486-486 (1982)
Studies on ω-Oxidation of Fatty Acids in vitro.
Kamei S, et al.
Journal of Biochemistry, 56(1), 72- 76 (1964)
Preparation of Macrocyclic Lactones by Depolymerization1.
Spanagel EW and Carothers WH.
Journal of the Chemical Society, 58(4), 654-656 (1936)
Chemo-enzymatic synthesis of 11-hydroxyundecanoic acid and 1, 11-undecanedioic acid from ricinoleic acid.
Jang HY, et al.
Green Chemistry, 18(4), 1089-1095 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service