C9525
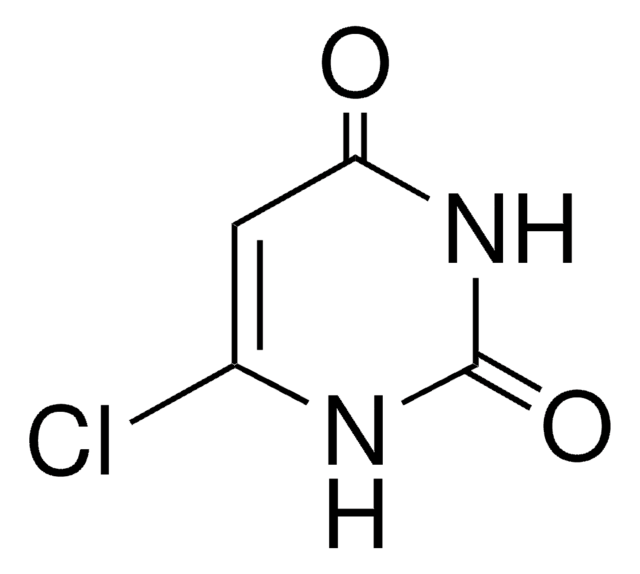
4-Chlorouracil
≥99%
Synonym(e):
4-Chloro-2,6-dihydroxypyrimidine, 6-Chloro-2,4-dihydroxypyrimidine, 6-Chlorouracil
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥99%
Form
solid
SMILES String
[H]N1C(Cl)=CC(=O)NC1=O
InChI
1S/C4H3ClN2O2/c5-2-1-3(8)7-4(9)6-2/h1H,(H2,6,7,8,9)
InChIKey
PKUFNWPSFCOSLU-UHFFFAOYSA-N
Anwendung
Reaction of 6-chlorouracil with 4-(dimethylamino)pyridine, 4-methylpyridine, and pyridin-4-yl-morpholine yielded pyridinium-substituted uracils as chlorides which were converted into pyridinium uracilates by deprotonation. These heterocyclic mesomeric betaines are cross-conjugated and thus possess separate cationic (pyridinium) and anionic (uracilate) moieties. Calculations and X-ray single crystal analyses may be used to characterize these systems and to compare the salts with the betaines.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.