C9231
Carbenicillin Dinatrium
meets USP testing specifications
Synonym(e):
Carbenicillin Dinatriumsalz, α-Carboxybenzylpenicillin Dinatriumsalz
About This Item
Agentur
USP/NF
meets USP testing specifications
Qualitätsniveau
Form
solid
Löslichkeit
H2O: 50 mg/mL
Wirkungsspektrum von Antibiotika
Gram-negative bacteria
Gram-positive bacteria
Anwendung(en)
pharmaceutical (small molecule)
Wirkungsweise
cell wall synthesis | interferes
Lagertemp.
2-8°C
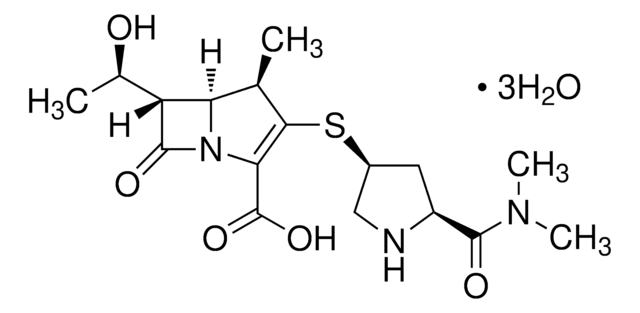
SMILES String
[Na+].[Na+].CC1(C)S[C@@H]2[C@H](NC(=O)C(C([O-])=O)c3ccccc3)C(=O)N2[C@H]1C([O-])=O
InChI
1S/C17H18N2O6S.2Na/c1-17(2)11(16(24)25)19-13(21)10(14(19)26-17)18-12(20)9(15(22)23)8-6-4-3-5-7-8;;/h3-7,9-11,14H,1-2H3,(H,18,20)(H,22,23)(H,24,25);;/q;2*+1/p-2/t9?,10-,11+,14-;;/m1../s1
InChIKey
RTYJTGSCYUUYAL-YCAHSCEMSA-L
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Antimikrobielles Spektrum: aktiv gegen grampositive und gramnegative Bakterien
Hinweis zur Analyse
Sonstige Hinweise
Signalwort
Danger
H-Sätze
P-Sätze
Gefahreneinstufungen
Resp. Sens. 1 - Skin Sens. 1
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Analysenzertifikate (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.