Quantification of Infliximab in Human Serum by LC-MS/MS Using A Full-Length Stable Isotope Labeled Internal Standard
Kevin Ray, Pegah R Jalili
MilliporeSigma, St. Louis
Infliximab, a chimeric monoclonal antibody, is used to treat rheumatoid arthritis, psoriatic arthritis, Crohn’s disease, and other autoimmune diseases. Clinical responses are different among patients due to inadequate amount of drug circulating in the blood. Therefore, there is a growing demand for reliable LC-MS/MS assays to support quantification of serum Infliximab in clinical applications. The accurate quantitation of Infliximab is enabled by early introduction of an internal standard that behaves identically to the native target protein throughout the analytical workflow. We have developed and characterized a full-length stable isotope labeled Infliximab internal standard and demonstrate its use to achieve sensitive, accurate, and reproducible quantification of serum Infliximab in an LC-MRM assay.
Methods
SIL-Infliximab was expressed in CHO cells which were grown in serum-free medium enriched with 13C6 15N4 Arg and 13C6 15N2 Lys. The SIL-Infliximab was analyzed at the intact protein level and after trypsin digestion. Intact mass analysis (SEC-MS) was used to confirm the amino acid composition of the protein and level of glycosylation. The sequence and isotope incorporation were determined at the peptide level after trypsin digestion.
For quantification, samples were prepared by spiking 25 μg/mL of SIL-Infliximab as an internal standard into human serum containing 0.5 - 100 μg/mL of Infliximab target antibody. Samples were precipitated by adding saturated ammonium sulfate, reconstituted with 50 mM ammonium bicarbonate, and digested using trypsin. Tryptic peptides were separated on a Supelco BIOshell A160 Peptide C18, 2.7 μM fused core particle column; 10 cm x 500 μM. Detection was performed in MRM mode on Sciex QTRAP 5500 system. Transitions of four unique Infliximab peptides, GLEWVAEIR, SINSATHYAESVK, YASESMSGIPSR, and DILLTQSPAILSVSPGER, were monitored.
LC-MS/MS Method for Infliximab

Figure 1. Extracted ion chromatogram (XIC) of four Infliximab-specific peptides.
SEC-MS Analysis

Figure 2. UV trace and deconvoluted mass spectra resulting from SECMS analysis of the reduced SIL-Infliximab standard. Theoretical molecular weights assume 100% isotopic incorporation and unreduced intrachain disulfide bonds.
Isotopic Incorporation of SIL-Infliximab

Figure 3. Incorporation of 13C6 15N4 labeled arginine in two unique surrogate peptides liberated from SIL-Infliximab was > 99%.
Binding Kinetics of SIL-Infliximab vs Remicade™

Figure 4. Binding curves for SIL-Infliximab and authentic Remicade antibody. TNF-α binding of SIL-Infliximab was equivalent to the therapeutic antibody.
Quantitation of Infliximab in Human Serum
Calibration Curves for Two HC Infliximab Specific Peptides

GLEWVAEIR (HC)
Calibration Curves for Two LC Infliximab Specific Peptides
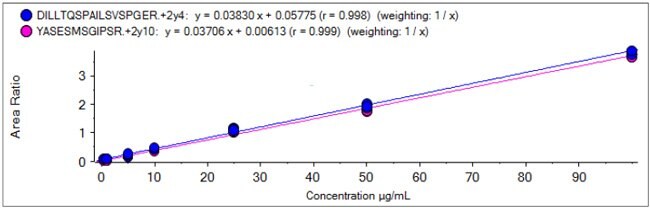
YASESMSGIPSR (LC)
Summary
- Stable isotope labeled full-length SIL-Infliximab antibody has been produced with high purity and isotopic incorporation >99%.
- We demonstrate that the use of a full length SIL-Infliximab internal standard enables sensitive, accurate, and reproducible quantification Infliximab in human serum*.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?