Assay Procedure for Lipase
Principle
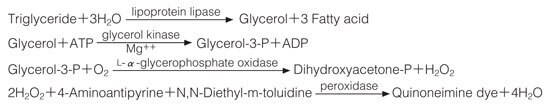
The appearance of quinoneimine dye is measured at 545 nm by spectrophotometry.
Unit definition
One unit causes the formation of one micromole of glycerol (half a micromole of quinoneimine dye) per minute under the conditions described below.
Method
Reagents
| A. Olive oil emulsion | :Sonicate the mixture of 5.0 g of olive oil[reagent grade (highly refined, low acidity)]and 5.0 mL of 5.0% Triton X-100 solution (B) for 10 minutes (20 KHz). To the oil emulsion, add 25 mL of 4.0% BSA solution (C) and 15 mL of 0.1M Kphosphate buffer, pH 7.0 (D), and mix. (Should be prepared freshly) |
| B. Triton X-100 solution | :5.0% (5.0 mL Triton X-100 detergent/100 mL of H2O) |
| C. BSA solution | :4.0%[4.0 g bovine serum albumin/100 mL of H2O] |
| D. K-phosphate buffer, pH 7.0 | :0.1 M |
| E. TCA solution | :0.2 M (33 g trichloroacetic acid/1,000 mL of H2O) |
| F. MES-NaOH buffer | :50 mM MES buffer, pH 6.5[Dissolve 9.76 g of 2-(N-morpholino)-ethanesulfonic acid (MW=195.23) in ca. 850 mL of H2O and, after adjusting the pH to 6.5 with 5.0 N NaOH, fill up to 1,000 mL with H2O] |
| G. Color developing reagent | :Dissolve the following chemicals and enzymes into 200 mL of 50 mM MES buffer (F) in the following order: |
| 4.0 mL 0.04 mL 4.0 mg 24.2 mg 40.7 mg 200 units 500 units 300 units | Triton X-100 solution (B) N,N-Diethyl-m-toluidine (Stir until completly dissolved) 4-Aminoantipyrine ATP・Na2・3H2O MgCl2・6H2O Glycerol kinase L-α-Glycerophosphate oxidase Peroxidase (Purpurogallin units) |
| (Stable for one week if stored at 4 ℃ in an amber bottle) | |
| H. Enzyme diluent | :20 mM K-phosphate buffer, pH 7.5 containing 2.0 mM MgCl2 and 0.5 mM EDTA-Na3 |
Procedure
(1st step)
- Pipette 2.0 mL of olive oil emulsion (A) into a test tube and equilibrate at 37 ℃ for about 5 minutes.
| Concentration in assay mixture | |
|---|---|
| K-Phosphate buffer | 29.1 mM |
| Olive oil | 90.9 mg/mL |
| MgCl2 | 0.18 mM |
| Triton X-100 | 9.1 % |
| EDTA | 45 μM |
| BSA | 1.8 % |
- Add 0.2 mL of the enzyme solution* and mix.
- After exactly 15 minutes at 37 ℃, add 2.0 mL of TCA solution (E) to stop the reaction and remove the precipitate by filtration through filter paper (Toyo-Roshi No.131 or Whatman No.42).
(2nd step)
- Pipette 0.05 mL of the filtrate thus obtained into a test tube.
- Add 3.0 mL of color developing reagent (G) and incubate at 37 ℃ for 15 minutes.
- Measure the optical density at 545 nm against water (OD test).
At the same time, prepare the blank by first mixing 2.0 mL of the olive oil emulsion (A) after 15 min incubation at 37 ℃ with 2.0 mL of TCA solution, followed by the addition of the enzyme solution (first step). By using the filtrate obtained from the mixture, carry out the 2nd step using the same procedure as the test and measure the optical density at 545 nm (OD blank).
* Dissolve the enzyme preparation in ice-cold enzyme diluent (H) and dilute to 0.4-1.2 U/mL with the same buffer, immediately before assay.
Calculation
Activity can be calculated by using the following formula:
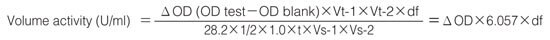
Weight activity (U/mg)=(U/mL)×1/C
| Vt-1 | :Total volume in 1st step (4.2 mL) |
| Vt-2 | :Total volume in 2nd step (3.05 mL) |
| Vs-1 | :Sample volume (0.2 mL) |
| Vs-2 | :Sample volume in 2nd step (0.05 mL) |
| 28.2 | :Millimolar extinction coefficient of quinoneimine dye under the assay condition (F/micromole) |
| 1/2 | :Factor based on the fact that one mole of H2O2 produces half a mole of quinoneimine dye |
| 1.0 | :Light path length (cm) |
| t | :Reaction time in 1st step (15 minutes) |
| df | :Dilution factor |
| C | :Enzyme concentration in dissolution (c mg/mL) |
This procedure is for informational purposes.
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?