C9409
Creatinine Deiminase microbial
lyophilized powder, ≥25 units/mg protein
Sinónimos:
Creatinine Deaminase
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Número de CAS:
EC Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Productos recomendados
form
lyophilized powder
Quality Level
specific activity
≥25 units/mg protein
mol wt
~260 kDa
composition
Protein, ≥15% biuret
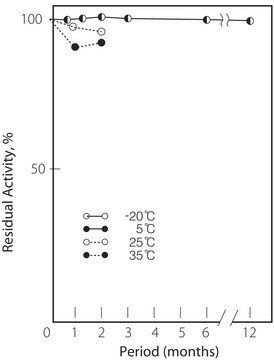
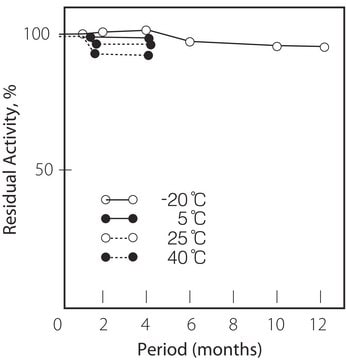
storage temp.
−20°C
Application
Creatinine Deiminase microbial has been used:
- to immobilize aminosilylated glass beads based biosensor for ammonia/ammonium and creatinine detection in urine
- in creating creatinine-sensing membrane for biophysical studies
- to investigate the bioelectronic tongue for the simultaneous determination of urea, creatinine and alkaline ions in clinical samples
Creatinine deiminase has been used in a study to assess the application of a creatinine-sensitive biosensor for hemodialysis control. Creatinine deiminase has also been used in a study to investigate the bioelectronic tongue for the simultaneous determination of urea, creatinine and alkaline ions in clinical samples.
Biochem/physiol Actions
Creatinine deiminase catalyzes the hydrolysis of creatinine to methylhydantoine and ammonia.
Physical properties
Isoelectric point : 4.4
Michaelis constant : 3.5 x 10‾3M (Creatinine)
Structure : 6 subunits per mol of enzyme
Inhibitors : Ag+,Hg++, o-phenanthroline,monoiodoacetate
Optimum pH : 8.5 – 9.5
Optimum temperature : 65 – 75°C
pH Stability : pH 7.0 – 11.0 (30°C, 20hr)
Thermal stability : Below 65°C (pH 7.5, 1hr)
Michaelis constant : 3.5 x 10‾3M (Creatinine)
Structure : 6 subunits per mol of enzyme
Inhibitors : Ag+,Hg++, o-phenanthroline,monoiodoacetate
Optimum pH : 8.5 – 9.5
Optimum temperature : 65 – 75°C
pH Stability : pH 7.0 – 11.0 (30°C, 20hr)
Thermal stability : Below 65°C (pH 7.5, 1hr)
Unit Definition
One unit will hydrolyze 1.0 μmole of creatinine to N-methylhydantoin and NH3 per min at pH 7.5 at 37 °C in a coupled system with L-glutamic dehydrogenase.
Physical form
Lyophilized powder containing mannitol as stabilizer
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Anne K Bendt et al.
Archives of microbiology, 181(6), 443-450 (2004-05-19)
In order to utilize different nitrogen sources and to survive situations of nitrogen limitation, microorganisms have developed several mechanisms to adapt their metabolism to changes in the nitrogen supply. In this communication, the use of creatinine as an alternative nitrogen
Hybrid biosensors for clinical analysis and fermentation control.
I Karube
Methods in enzymology, 137, 131-138 (1988-01-01)
T W Esders et al.
The Journal of biological chemistry, 260(7), 3915-3922 (1985-04-10)
Creatinine iminohydrolase (EC 3.5.4.21), which catalyzes hydrolysis of creatinine to N-methylhydantoin and ammonia, was purified from Flavobacterium filamentosum. The average molecular weight of the purified enzyme was 272,480, and the subunit molecular weight was 44,300. Extensive specificity studies indicated that
[Immobilized enzyme reactors--their use in chemiluminescence detection].
M Tabata et al.
Tanpakushitsu kakusan koso. Protein, nucleic acid, enzyme, 31(3), 220-229 (1986-03-01)
Hsiao-Chung Tsai et al.
Analytical biochemistry, 334(1), 183-192 (2004-10-07)
An optical array biosensor encapsulated with hydrolase and oxidoreductase using sol-gel immobilization technique has been fabricated for simultaneous analysis and screening of multiple samples to determine the presence of multianalytes which are clinically important in relation to renal failure. Urease
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico