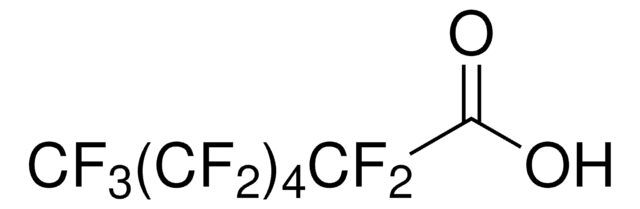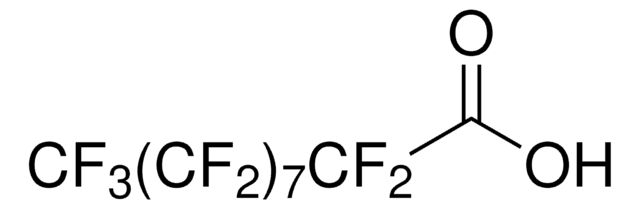52411
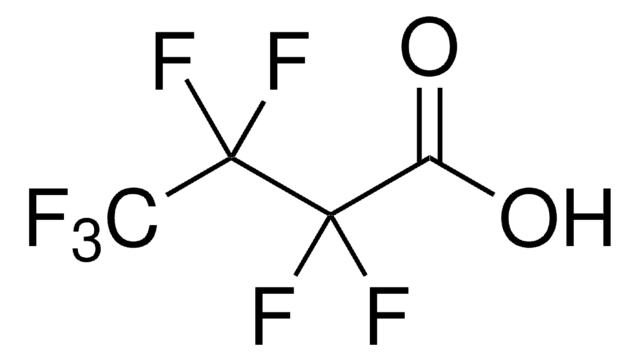
Heptafluorobutyric acid
suitable for ion chromatography, ≥99.5% (GC)
Synonym(s):
Edman Reagent No. 3, HFBA, Perfluorobutyric acid
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Linear Formula:
CF3CF2CF2COOH
CAS Number:
Molecular Weight:
214.04
Beilstein:
1426882
EC Number:
MDL number:
UNSPSC Code:
12000000
PubChem Substance ID:
NACRES:
NB.21
Recommended Products
vapor density
7 (vs air)
Quality Level
vapor pressure
~10 mmHg ( 25 °C)
description
anionic
Assay
≥99.5% (GC)
form
liquid
shelf life
limited shelf life, expiry date on the label
technique(s)
ion chromatography: suitable
refractive index
n20/D 1.3 (lit.)
bp
120 °C/755 mmHg (lit.)
Looking for similar products? Visit Product Comparison Guide
Application
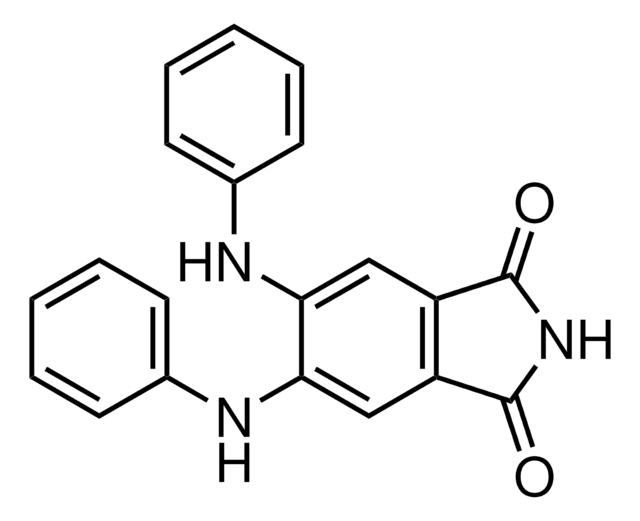
Heptafluorobutyric acid (HFBA) may be used to cleave glycylserine bonds, in an experimental protocol done in order to sequence glycine-rich protein from lizard claw.
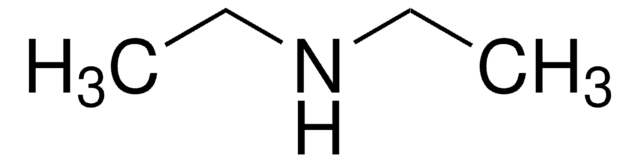
Linkage
Visit the IC Portal to learn more
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1A
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sequence of a glycine-rich protein from lizard claw: unusual dilute acid and heptafluorobutyric acid cleavages.
Inglis A
Proteins: Structure, Function, and Genetics, 757-764 (1987)
Yuko Murakami et al.
Organic & biomolecular chemistry, 12(48), 9887-9894 (2014-10-31)
Desmosine-CH2, an analog of the elastic tissue degradation biomarker desmosine, can be regarded as a potential internal standard for precise quantification of desmosines by LC-MS/MS. In this study, the chemical synthesis of desmosine-CH2 was completed in 22% overall yield in
Ulrika Eriksson et al.
Environmental science and pollution research international, 20(11), 7940-7948 (2013-04-17)
Diet and drinking water are suggested to be major exposure pathways for perfluoroalkyl substances (PFASs). In this study, food items and water from Faroe Islands sampled in 2011/2012 were analyzed for 11 perfluoroalkyl carboxylic acids (PFCAs) and 4 perfluoroalkane sulfonic
A Hagenaars et al.
Chemosphere, 82(5), 764-772 (2010-11-30)
Perfluorinated compounds (PFCs) are a group of anthropogenic chemicals containing diverse functional groups and chain lengths. They are known to be persistent and bioaccumulative explaining their worldwide environmental presence. The toxicological information on these chemicals is still incomplete and insufficient
Gerold Jerz et al.
Journal of chromatography. A, 1344, 42-50 (2014-04-29)
Betacyanins, red-violet plant pigments, were fractionated by ion-pair high-speed countercurrent chromatography (IP-HSCCC) from leaves extract of Iresine lindenii Van Houtte, an ornamental plant of the family Amaranthaceae. An HSCCC solvent system consisting of TBME-1-BuOH-ACN-H2O (1:3:1:5, v/v/v/v) was applied using ion-pair
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service