45391
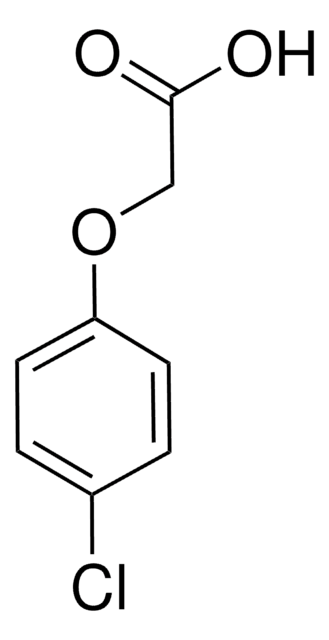
4-Chlorophenoxyacetic acid
PESTANAL®, analytical standard
Synonym(s):
4-CPA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H7ClO3
CAS Number:
Molecular Weight:
186.59
Beilstein:
1211804
EC Number:
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
neat
SMILES string
OC(=O)COc1ccc(Cl)cc1
InChI
1S/C8H7ClO3/c9-6-1-3-7(4-2-6)12-5-8(10)11/h1-4H,5H2,(H,10,11)
InChI key
SODPIMGUZLOIPE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Recommended products
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
Legal Information
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G Carbonara et al.
Farmaco (Societa chimica italiana : 1989), 56(10), 749-754 (2001-11-23)
2-(4-Chloro-phenoxy)propanoic and 2-(4-chloro-phenoxy)butanoic acids are compounds known to block chloride membrane conductance in rat striated muscle by interaction with a specific receptor. In the present study, a series of chiral analogues has been prepared and tested to evaluate the influence
K T Ranjit et al.
Environmental science & technology, 35(7), 1544-1549 (2001-05-12)
The photocatalytic degradation of p-chlorophenoxyacetic acid has been investigated in oxygenated aqueous suspensions of lanthanide oxide-doped TiO2 photocatalysts. Complete mineralization was achieved. The enhanced degradation is attributed to the formation of Lewis acid-base complex between the lanthanide ion and the
Birame Boye et al.
Environmental science & technology, 36(13), 3030-3035 (2002-07-30)
The herbicide 4-chlorophenoxyacetic acid (4-CPA) has been degraded in aqueous medium by advanced electrochemical oxidation processes such as electro-Fenton and photoelectro-Fenton with UV light, using an undivided cell containing a Pt anode. In these environmentally clean methods, the main oxidant
Y Alicigüzel et al.
Redox report : communications in free radical research, 6(3), 153-154 (2001-08-29)
To investigate the possible role of oxygen free radicals and oxidant stress in the toxic effects of phenoxyherbicides, we studied the in vitro effect of 4-chlorophenoxyacetic acid (4-CPA) on various human erythrocyte antioxidant enzymes, namely glucose-6-phosphate dehydrogenase, catalase, selenium-dependent glutathione
Ting-Fu Jiang et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 22(6), 811-814 (2006-06-15)
A new, simple and rapid capillary electrophoresis (CE) method, using hexadimethrine bromide (HDB) as electroosmotic flow (EOF) modifier, was developed for the identification and quantitative determination of four plant hormones, including gibberellin A3 (GA3), indole-3-acetic acid (IAA), alpha-naphthaleneacetic acid (NAA)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service